International Journal of Veterinary Science and Research
Wound healing by brand new product
Lee Jae Bong1, Cho Seong Keun2, Lim Jong So3* and Kang Kyung Soo4*
2Professor, Department of Animal Science, College of Natural Resources & Life Science, Pusan National University, Miryang, Gyeongnam 50463, Korea
3Adjunct Professor, Department of Bio Life Sciences, Shingu College, Seongnam, Gyeonggi-Do, 13174, Korea
4Assistant Professor, Department of Bio Life Sciences, Shingu College, Seongnam, Gyeonggi-Do, 13174, Korea
Kang Kyung Soo, Assistant Professor, Department of Bio Life Sciences, Shingu College, Seongnam, Gyeonggi-Do, 13174, Korea, E-mail: kks8055@shingu.ac.kr
Cite this as
Bong LJ, Keun CS, So LJ, Soo KK (2023) Wound healing by brand new product. Int J Vet Sci Res 9(1): 005-012. DOI: 10.17352/ijvsr.000131Copyright License
© 2023 Bong LJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Eggshell Membrane (ESM) has been used as an alternative natural bandage to cure wounds and is available in large quantities from egg industries. This study is based on the use of natural resources for skin tissue reconstruction. It needs to understand regeneration of tissue with Extracellular Matrix (ECM).
Wound healing is a complicated and continuous recovery process of damaged tissues by replacing dysfunctional injured cellular structures. The wound healing process recruits some different phases: the first phase for hemostasis, the second phase for inflammation, the third phase for proliferation, and last phases for maturation/tissue remodeling. Each process needs an appropriate surrounding to achieve accelerated healing. Because the skin is in contact with the outside, injuries occur and therefore the surface is often injured. Due to the different types of wounds, as well as the advancement in medical technology, various products have been developed to repair different skin lesions.
We investigate the wound healing effects which are measured by dividing into the Exture® group, the commercially available wound dressing group (positive control), and the untreated group (negative control). We induced wounds and measured the healing process for 20 days for a comparison experiment of collagen membrane wound healing.
As for the wound, the decrease in wound area using external photographs, the healing process within the tissue, and the immune response were measured through hematology analysis. We confirmed some differences through comparison of blood cell analysis and wound healing process but did not find statistical significance. There was no significant difference between treated collagen wound dressing film and commercial wound dressing film.
Thus, this study reveals that the possibility of use natural source-based wound healing products brings sufficient wound healing results.
Introduction
In addition to covering the body’s surface, the skin is the largest organ that performs essential functions in the body’s immunity, thermoregulation, and protection [1]. However, because the skin is in contact with the outside, injuries occur and therefore the surface is often injured [2]. The occurrence of wounds is unpredictable, and the healing process is complex and diversified [3].
For a wound to healing, it undergoes a process of tissue growth and regeneration consisting of hemostasis, proliferation, migration, inflammation, and tissue modeling [4]. Traditionally, for wound healing, humans have used bioactive compounds for many types of wound management [5]. Skin regeneration is a complex of cell and other component cooperation comprising interfaces between in immune and connective tissue cells [6]. The early stage of the wound healing process is very important, i.e., it needs to be provided with an Extracellular Matrix (ECM) basis for cell regeneration and reduced scar formation [5]. An acellular ECM is a protein-rich matrix that is essential for cell adhesion and structural support [7]. The microstructures of native ECM scaffolds are used in tissue engineering and promote cell endo growth, ECM deposition, and neo-tissue formation [5].
The integument is a complex coordinated wound-healing cascade to maintain homeostasis [8]. When healing skin wounds, the longer it takes for the wound to heal, the more the disease penetrates the body and has a pathologically bad effect. Implantation of ECM into the wound skin to reduce the duration of wound healing can accelerate wound healing [9]. Incisional or surgical wounds do not lead to fibrosis and heal through connective tissue and epithelial regeneration [10].
Cells and the microenvironment form a comprehensive network of tissue repair, cell proliferation, cell differentiation, migration, and apoptosis. In addition, the extracellular matrix (ECM), which is a complex network of fibrous proteins, carbohydrates, and glycoproteins contained in cells, is an important factor in tissue regeneration [11].
The final stage of wound healing is remodeling into scar tissue. The formation of new stromal tissue is also associated with the initiation of re-epithelialization and is produced by local keratinocytes at the wound margin and epithelial stem cells in sweat glands or hair follicles [12].
When the skin is damaged, blood vessels constrict, platelets are activated to coagulate, and bleeding stops [13]. Fibrin helps white blood cells infiltrate. Neutrophils migrate to the site of injury and are the first line of defense against infection [14]. Among the immune cells involved in the repair process of damaged skin, neutrophils are rapidly recruited by about 50% of all cells in the damaged area within 24 hours after injury, and are called “first responders”[3].
The biomimetic scaffolds have been used to assist in wound healing and skin reconstruction [15]. Eggshell Membrane (ESM) has been used as a biomaterial that promotes cell growth for skin wound healing. Water-soluble ESM could promote the synthesis of type III collagen in mouse skin and greatly improve the elasticity of human skin and reduce facial wrinkles [16]. The eggshell membrane is composed of a two-layered structure consisting of the inner and outer shell membranes. The fiber thickness of the shell membrane was 0.1 μm - 3 μm and the inner shell membrane was 15 μm - 26 μm thick, and the thickness of the whole layer was not calcified. The outer film, with individual fiber thicknesses in the range of 1 μm - 7 μm and total layer thickness of 50 μm - 70 μm, is calcified and infiltrated into the inner surface of the ES [17,18]. The average thickness of the double-layer ESM is about 70 μm.
Natural biomimetic scaffolds are suitable for wound healing. This scaffold modulates collagen, elastin, glycosaminoglycans, proteoglycans and connective tissue glycoproteins that make up skin tissue structure and activate important cellular pathways [19].
Ovozen Co., Ltd. has developed a new biomaterial replacement product using collagen extracted from eggs which is Exture®. The product produced using ESM is known to promote cell growth and increase adhesion to show excellent efficacy in wound healing [20].
The characterization of the Exture® is very similar to a natural eggshell membrane. This product focused on cell culture, material production and 3D culture system [21].
The porous structure of ESM is widely used in biological applications because it is easily available for cell culture, biocompatible, free from contaminants, and is an eco-friendly material that functions by itself or after modification [20]. ESM is a protein-based fibrous tissue that is located between the egg shell and the egg white to prevent bacterial invasion and has similar functions to ECM components [22].
The structure of ESM consists of an inner knit network of thin fibers and an outer network of thick fibers. The composition of ESM is that in an organic matrix, 10% of the constituents are collagens I, V and X, which act as primary structural components, and 70% - 75% of the constituents are secondary, including osteopontin, keratin, proteoglycans and glycoproteins. It has a protein component [23,24].
The eggshell membrane is composed of a large number of amino acids and has many therapeutic applications in unprocessed or processed forms [25].
Contribution/Originality
This study is one of the unique studies which have investigated by comparing the wound dressing efficiency of the wound dressing test membrane using eggshell membrane, which is natural collagen, between the untreated group and the commercial membrane treated group. The study has also contributed to the existing literature concerning the wound dressing efficiency of a natural collagen.
Methodology
Wound healing
The experiment was divided into 3 groups. The wound healing effect was measured by dividing into the Exture® (Ovozen co., Ltd) film type group, the commercially available wound dressing group, and the untreated group. Each treatment group used 5 animals in the experiment.
In order to provide the same environment for each treatment group, sterilized gauze (Tegaderm; 3M Health Care, USA) is covered, and an elastic band (Cobanl; 3M Health Care, USA) is wrapped over it so as not to interfere with movement.
Observation of macroscopic wound changes
In order to observe the progress of wound healing, images were taken at a certain distance for each group using a digital camera (CAMEDIATM, Olympus Co., Tokyo, Japan) at intervals of 5 days from the start of wound induction, and the changes in the wound were visually observed.
Measure the area of the wound with a digital caliper (Mitutoyo Co., Kawasaki, Japan) to indicate it as an objective index.
Wound healing rate (%) = treatment occupancy by period (%) = Wo: Wound area immediately after wound induction Ui: Wound area on the measurement date Ub: Wound area on the previous measurement date Histological examination.
Animals
The subject SD rats were fasted for 24 hours before anesthesia, and water was supplied.
In all experimental groups, one hour before the induction of wounds, 30 µL of zoletyl 50 and Lumpun were mixed in a (1:2) ratio and anesthetized by intramuscular injection.
After disinfection by applying 10% povidine-iodine and 70% ethanol to the back, a fullthickness defect including the dermis was induced with a diameter of 2 cm × 2 cm using sterilized surgical scissors on the back.
Animal testing was approved by the Animal Experiment Ethics Committee of the Chonbuk National University Transmitted Infectious Disease Research Center (IACUC - JBNU 2021065).
The wound healing rate was calculated by the method suggested in previous studies 7,8 for the change in the wound.
After 20 days from the induction of the wound, the experimental animal is euthanized using ethyl ether according to the method by excessive administration of psychotropic drugs specified in the standards for laboratory animal management guidelines of Notification No. 889 of the Ministry of Health and Welfare.
Observation of microscopic wound changes
Wound healing rate (%) = Treatment share by period (%) = Wo: wound area immediately after wound induction Ui: wound area on the measurement date Ub: wound area on the previous measurement date Histological examination. After 20 days have elapsed since the induction of the wound, the experimental animals were euthanized using ethyl ether using ethyl ether according to the method by the overdose of psychotropic drugs specified in the guidelines for the management of laboratory animals of the Ministry of Health and Welfare Notice No. 88-9. Did it.
Tissue including the entire circular wound was collected, fixed in 10% neutral formalin solution for 24 hours, and a section passing through the center of the wound was taken, dehydrated, and embedded in a paraffin block.
Tissues were cut using a tissue microtome, attached to polylysine-coated slides, paraffin removed and hydrated, and subjected to Hematoxylin-Eosin staining and Masson’s trichrome staining.
Blood cell analysis
Blood is collected for use in blood cell analysis prior to the start of testing and at the end of animal testing.
Histopathology
Tissues containing the entire round wound were collected, fixed in 10% neutral formalin solution for 24 hours, and a section passing through the center of the wound was dehydrated and embedded in a paraffin block. Tissues were cut using a tissue sectioned and then attached to polylysine-coated slides to remove paraffin and hydrate. Then, Hematoxylin-Eosin staining, and Masson’s trichrome staining were performed. Statistical processing. All experimental results were expressed as mean values and standard deviations. The statistical significance test between the control group and the experimental group was compared by the student’s t - test and only those with p of 0.05 or less and 0.01 or less were considered significant.
Result
Wound closure evolution
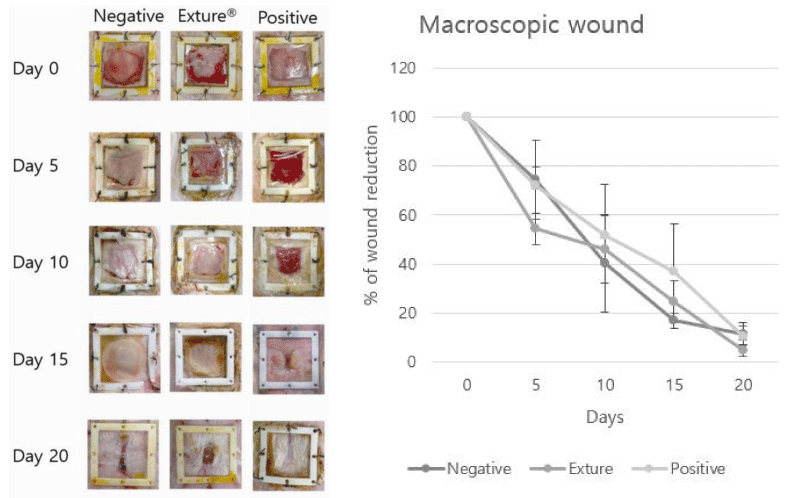
For this assessment, wound healing rate was measured by wound healing area (% of original wound area). On day 5, 10, 25, 20 post-surgery, animals from the control group showed a lower wound healing rate than the commercial and Exture® groups, which showed a reduction in the open area of the wound (100% to 4.8%). At this time point, no significant differences were also found between the commercial and Exture® groups (*p < 0.05) (Figure 1).
Looking at the healing effect according to the wound healing period of the different groups, the negative control group was healed from 100% to 11.2% and the positive control group showing 100% to 10.2% was confirmed Figure 1.
Evaluation of the reparative process.
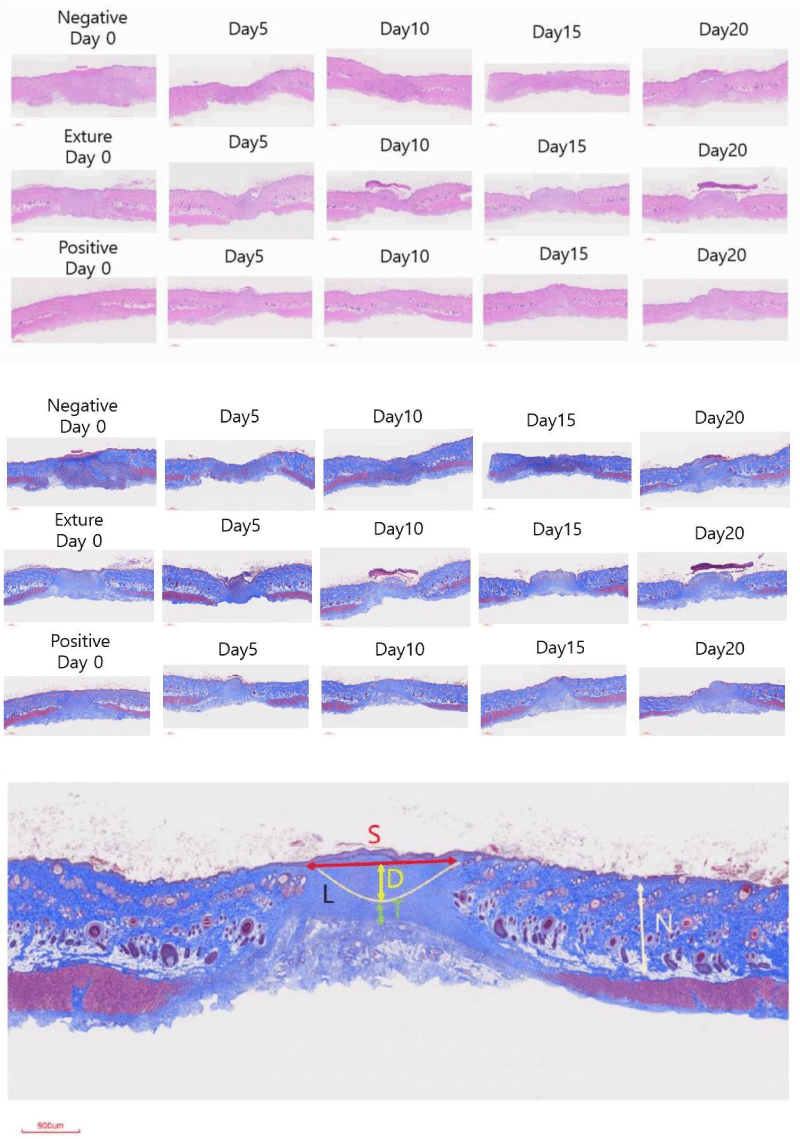
Five days after the intervention, all the animals showed a large non-epithelialized defect area and an inflammatory exudate covering the superficial zones. In general, in the wound area, granulation tissue with numerous blood vessels and signs of evident inflammatory and proliferative phases of reparative process were observed in panoramic histological images (Figure 2). The control group showed the greatest development and thickness of this tissue.
In groups negative control, Exture® and positive control, reduced granulation tissue formation with homogeneous thickness, fewer inflammatory cells, and induction of vasculature in the neo-formed tissue were observed (Figure 2).
The pattern of wound healing among those three different treatments reveals the difference in healing points. There are a lot of differences between the positive, Exture® groups and with negative group of expression pattern (Figure 3).
15th Days wound healing score of SCI shows that Exture® group are best wound healing point. However, Total of score of wound healing measurements are best in positive control.
Blood analysis
To survey inflammation and proliferation, blood is collected for use in blood chemistry analysis prior to the start of testing and at the end of animal testing.
The interpretation of the results of the blood analysis tested in this study can be interpreted according to the distribution of each blood in the blood and the percentage of the total blood [26].
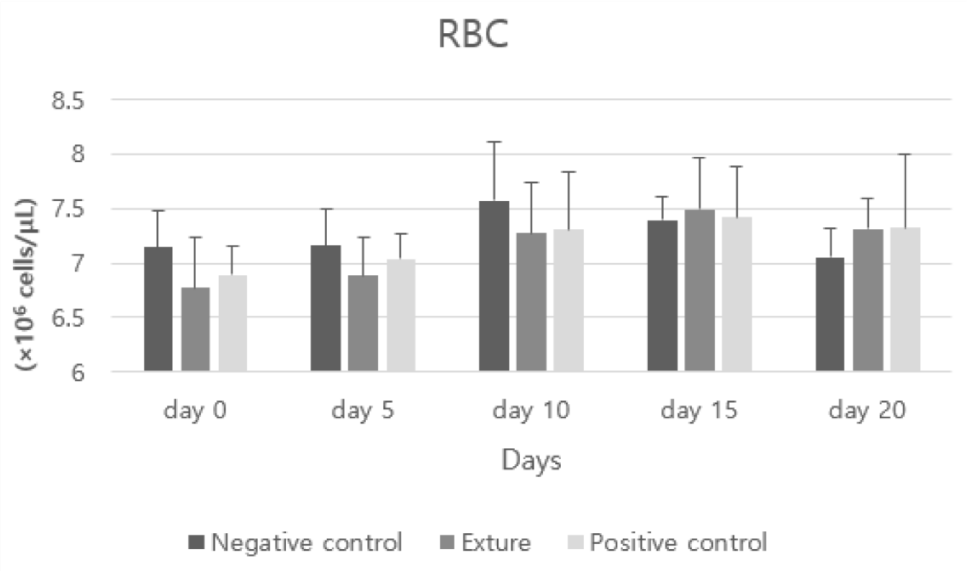
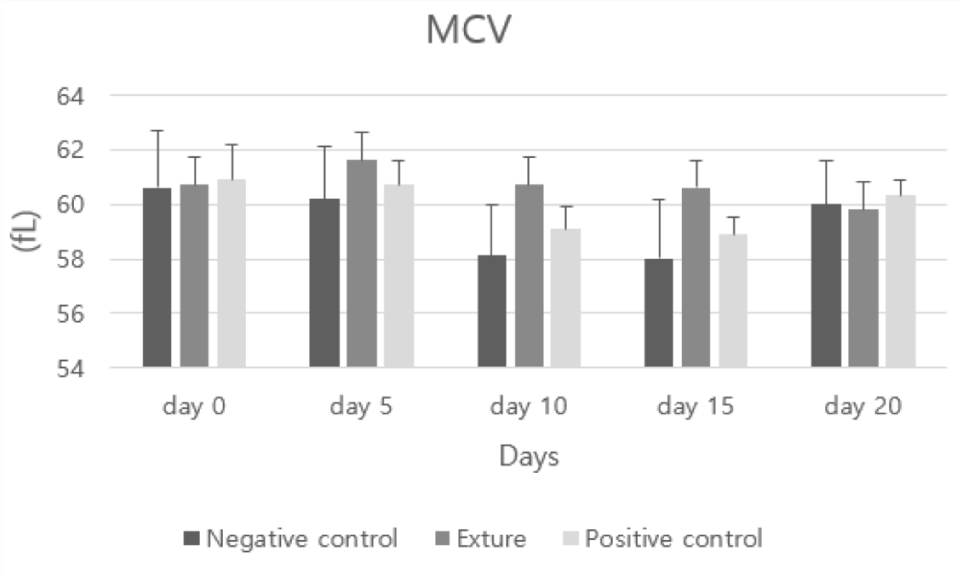
RBC (Red Blood Cell count) and MCV (mean corpuscular volume) figure was different in each group. But this difference was not very meaningful to estimate hemorrhage.
Red blood cells play a role in supplying oxygen used as a very important raw material to the body throughout the body.
In general, a high red blood cell count is often a sign of dehydration. In certain cases, it is also seen as a sign of an uncommon disease that results in excessive amounts of red blood cells in the bone marrow. A low red blood cell count is sometimes attributed to anemia, blood loss, hemorrhage, bone marrow disease or immune disease, and severe red blood cell destruction due to ingestion of poisons [26].
Hemoglobin has the property of binding oxygen to red blood cells and releasing the bound oxygen in the body where the partial pressure of oxygen is low. A high level of hemoglobin usually indicates a high red blood cell count and dehydration. Low levels can indicate anemia, hemorrhage, or iron deficiency.
MCV represents the mean corpuscular volume and is the average quantity of red blood cells. Therefore, a high MCV indicates a specific vitamin deficiency, and a low mean iron deficiency.
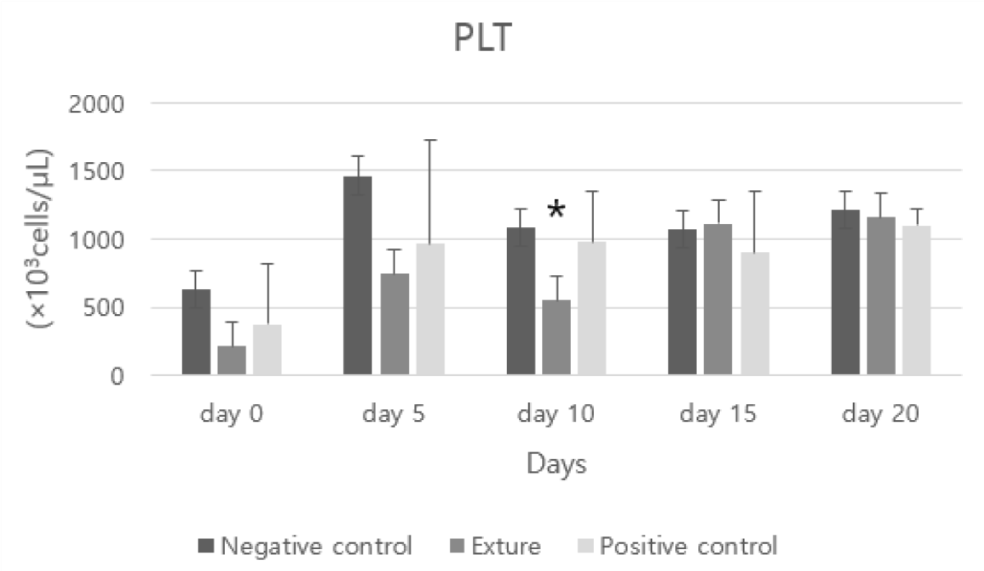
PLT represents the platelet and plays a role in preventing blood from leaking from blood vessels. In general, high platelet counts suggest bone marrow abnormalities and are associated with immune blood diseases. If the platelet count is low, blood may be leaking somewhere, or a huge amount of platelet destruction may be suspected due to a reaction with an immune disease or parasite [26].
At day 10 Exture®’s PLT (platelet) figure was lower than the other group. This means Exture® group’s hemostasis process was well progressed than the other groups.
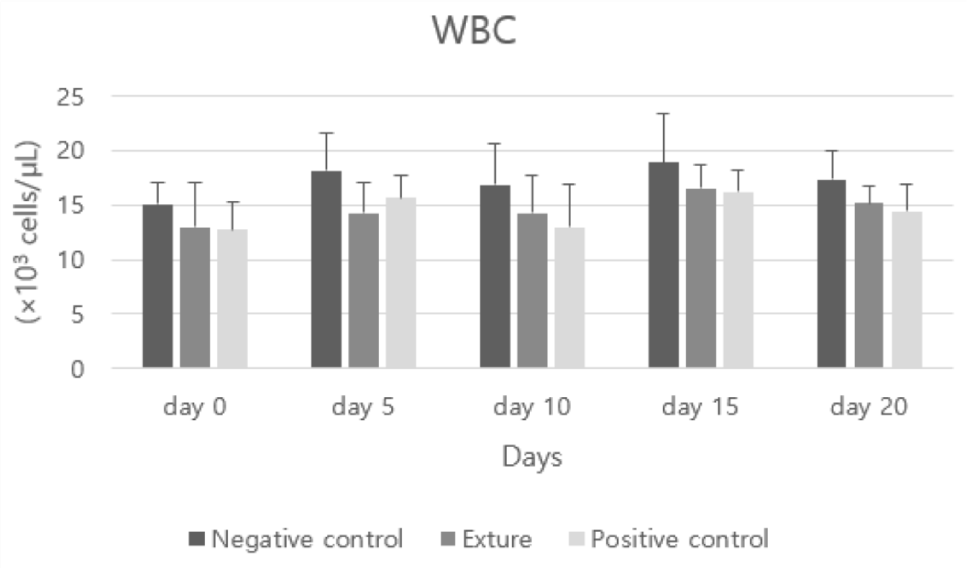
Negative control’s WBC (white blood cell count) higher figure was assumed to insufficient inflammation process which is need to proper immune response than the others Figures 4-7.
White blood cells fight germs that infect the body and respond when inflammation occurs.
An increased number of white blood cells may be observed in infection, inflammation, cancer or leukemia.
A low white blood cell count can be a sign of a viral infection, bone marrow abnormality, or severe infection or sepsis, and is concentrated at the site of infection. Since it does not circulate in the blood, the natural level is low [26].
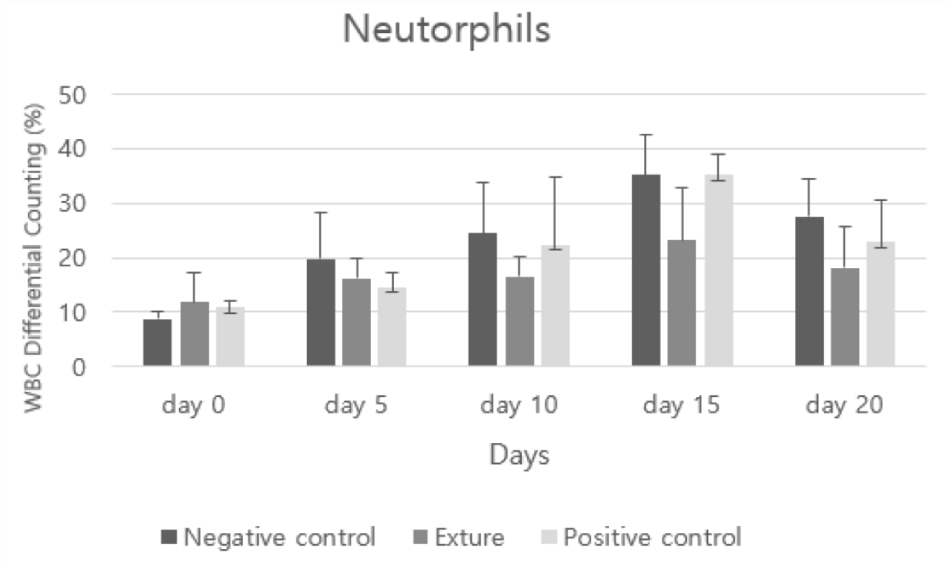
Segs represents segmental neutrophils, and a high number means infection somewhere, and a low number means sepsis. In addition, when neutrophils are concentrated at the site of infection or used immediately, they circulate less in the blood [26].
In analysis of neutrophils, after day 10 Exture®’s figure was lower than the others. It can be assumed to efficient phagocytosis process occurs in Exture® group (Figure 8).
LYM means lymphocytes and plays a role in fighting pathogens and produces antibodies to prevent infiltration of pathogens. Lymphocyte counts are elevated in the presence of infections, viral diseases, or certain cancers such as lymphosarcoma, while low levels indicate sepsis or infection with a virus affecting the bone marrow [26].
In analysis of LYM (lymphocytes), no significant difference was observed. It means all groups were not infected by bacteria or virus.
Mono means monocytes (monocytes) and removes germs like neutrophils. If the level is high, the complete disappearance of monocytes does not occur, indicating that there is an infection, and even if the level is 0, it does not mean that a specific disease has occurred [26].
In analysis of MONO (monocytes), at day 5,10 Days Exture® group’s figure was lower than the others. It can be assumed that innate immunity by macrophage is more accelerated than the others (Figures 9,10).
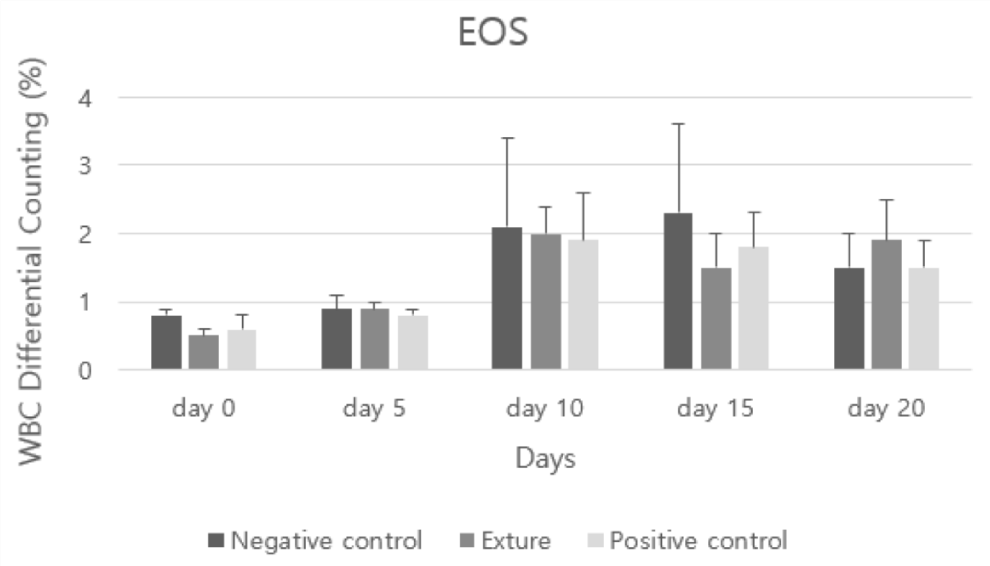
Eos means eosinophil (coral white blood cell) and is a white blood cell that responds to allergies or parasites. Even in normal blood, the coral white blood cell count may be 0, but it is generally not possible to have a low level [26].
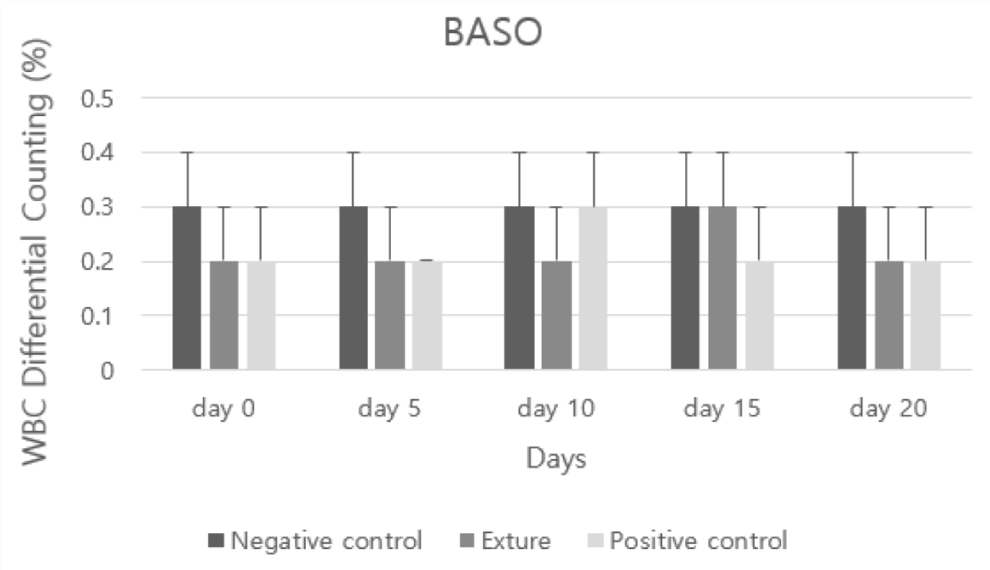
Baso represents basophils (basophilic white blood cells) and appears when infected with certain parasites, especially heartworm, and the level may be 0 even in normal blood samples, but it is impossible to get a completely low level [26].
In analysis of EOS (eosinophils) and BASO (basophils), all groups were not shown allergic response. But at day 0 and 5, negative control group was higher than the others (Figures 11,12).
Discussion
The main purpose of the wide spectrum of devices or treatments developed to promote wound healing is that this physiological process promotes wound closure as soon as possible and results in a functionally and aesthetically satisfactory scar [21]. For this purpose, the organism must be able to reduce cutaneous discontinuity by generating new granulation tissue in the wounded area. An epithelial barrier must be able to develop on this tissue, reaching continuity and avoiding re-openings through the formation of a resistant extracellular matrix. This results from cell proliferation, synthesis, and maturation of the extracellular matrix [25].
In general, the wound repair process occurs in almost all tissues after exposure to a destructive stimulus. It must be taken into consideration that, in this study, the physiological healing process was not compromised in any way. In this study, the figures of LYM, EOS, BASO shows all the animals used were healthy animals without pathologies that might have affected healing; therefore, the model showed a standard healing pattern, with characteristics of the two phases of the skin repair process (inflammation, proliferation) appearing in a progressive and orderly manner until the achievement of newly formed connective tissue with reduced cellularity and a dense and organized extracellular matrix. In blood analysis of this study, WBC (white blood cell count) figure and at day 5 and 10, negative control’s higher BASO (basophils) figure than the other groups indicate efficient inflammation and proliferation process was occurred in Exture® group and commercial group [26].
Other preclinical trials using modified collagen gel treatments versus untreated wounds have shown acute inflammatory cell and fibroblast recruitment, collagen I deposition, increased endothelial cells, upregulated vascular endothelial growth factor, and improved blood flow [26,27]. That was in accordance with our results (neutrophils, WBC, MONO), so we can assume that Exture® film treated wounds displayed increased neoangiogenesis, less inflammatory granulation tissue and accelerated proliferation. At day 0 and 5, PLT and BASO’s negative control’s figure was higher than the others. At day 5, PLT figure of Exture is lower than the others. It can be assumed that Exture®’s hemostasis process is more accelerated than the others. Negative control’s higher PLT and BASO figures were caused by excessive inflammation. Because basophil produce compounds histamine and serotonin that induce inflammation, heparin that prevents blood clotting [28-30].
Taking into account all the morphometric and histological results of this research, we can conclude that the Exture® group, compared to the untreated control group, produces faster wound closure and, at the same time, promotes a slight progression of the reparative process. But no significant difference was observed with commercial group. However, additional studies are needed to confirm the effects of this Exture® film in terms of a compromised healing process.
Author contributions
Conceptualization, Lee Jae Bong and kang Kyung Soo; writing-original draft preparation, Cho, Seong Keun, Lim jong so and kang Kyung Soo; writing-review and editing, Cho Seong Keun and kang Kyung Soo; supervision and project administration, Cho Seong Keun and Lee Jae Bong; funding acquisition, Lee Jae Bong All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a 2-Year Research Grant of Pusan National University.
- Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002 Jul-Aug;12(4):390-9; quiz 400-1. PMID: 12095893.
- Al Aboud AM, Manna B. Wound Pressure Injury Management, in StatPearls. 2022: Treasure Island (FL).
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014 Dec 3;6(265):265sr6. doi: 10.1126/scitranslmed.3009337. PMID: 25473038; PMCID: PMC4973620.
- Fitridge R, Thompson M, editors. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. Adelaide (AU): University of Adelaide Press; 2011. PMID: 30484990.
- Ryall C, Duarah S, Chen S, Yu H, Wen J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics. 2022 May 17;14(5):1072. doi: 10.3390/pharmaceutics14051072. PMID: 35631658; PMCID: PMC9143175.
- Krafts KP. Tissue repair: The hidden drama. Organogenesis. 2010 Oct-Dec;6(4):225-33. doi: 10.4161/org.6.4.12555. PMID: 21220961; PMCID: PMC3055648.
- Xing H, Lee H, Luo L, Kyriakides TR. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. 2020 Sep-Oct;42:107421. doi: 10.1016/j.biotechadv.2019.107421. Epub 2019 Aug 2. PMID: 31381963; PMCID: PMC6995418.
- Childs DR, Murthy AS. Overview of Wound Healing and Management. Surg Clin North Am. 2017 Feb;97(1):189-207. doi: 10.1016/j.suc.2016.08.013. PMID: 27894427.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004 Jan 1;9:283-9. doi: 10.2741/1184. PMID: 14766366.
- Miguel SP, Sequeira RS, Moreira AF, Cabral CSD, Mendonça AG, Ferreira P, Correia IJ. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur J Pharm Biopharm. 2019 Jun;139:1-22. doi: 10.1016/j.ejpb.2019.03.010. Epub 2019 Mar 7. PMID: 30853442.
- Vargas J, Uribe-Escamilla R, Alfaro-Rodríguez A. Proteinas inhibidoras de la regeneracion neuritica en la matriz extracelular: estructura, interacciones moleculares y sus funciones. Mecanismos del balance extracelular [Inhibitory proteins of neuritic regeneration in the extracellular matrix: structure, molecular interactions and their functions. Mechanisms of extracellular balance]. Rev Invest Clin. 2013 Jul-Aug;65(4):336-48. Spanish. PMID: 24304735.
- Fusenig NE, Limat A, Stark HJ, Breitkreutz D. Modulation of the differentiated phenotype of keratinocytes of the hair follicle and from epidermis. J Dermatol Sci. 1994 Jul;7 Suppl:S142-51. doi: 10.1016/0923-1811(94)90045-0. PMID: 7999672.
- Clark RA. Fibrin is a many splendored thing. J Invest Dermatol. 2003 Nov;121(5):xxi-xxii. doi: 10.1046/j.1523-1747.2003.12575.x. PMID: 14708590.
- Wilgus TA, Roy S, McDaniel JC. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv Wound Care (New Rochelle). 2013 Sep;2(7):379-388. doi: 10.1089/wound.2012.0383. PMID: 24527354; PMCID: PMC3763227.
- Arasteh S, Khanjani S, Golshahi H, Mobini S, Jahed MT, Heidari-Vala H, Edalatkhah H, Kazemnejad S. Efficient Wound Healing Using a Synthetic Nanofibrous Bilayer Skin Substitute in Murine Model. J Surg Res. 2020 Jan;245:31-44. doi: 10.1016/j.jss.2019.07.017. Epub 2019 Aug 7. PMID: 31400575.
- Ohto-Fujita E, Shimizu M, Sano S, Kurimoto M, Yamazawa K, Atomi T, Sakurai T, Murakami Y, Takami T, Murakami T, Yoshimura K, Hasebe Y, Atomi Y. Solubilized eggshell membrane supplies a type III collagen-rich elastic dermal papilla. Cell Tissue Res. 2019 Apr;376(1):123-135. doi: 10.1007/s00441-018-2954-3. Epub 2018 Nov 17. PMID: 30448901.
- Bellairs R, Boyde A. Scanning electron microscopy of the shell membranes of the hen's egg. Z Zellforsch Mikrosk Anat. 1969;96(2):237-49. doi: 10.1007/BF00338771. PMID: 5772036.
- Hincke MT, Nys Y, Gautron J, Mann K, Rodriguez-Navarro AB, McKee MD. The eggshell: structure, composition and mineralization. Front Biosci (Landmark Ed). 2012 Jan 1;17(4):1266-80. doi: 10.2741/3985. PMID: 22201802.
- Marques CF, Diogo GS, Pina S, Oliveira JM, Silva TH, Reis RL. Collagen-based bioinks for hard tissue engineering applications: a comprehensive review. J Mater Sci Mater Med. 2019 Mar 6;30(3):32. doi: 10.1007/s10856-019-6234-x. PMID: 30840132.
- Sah MK, Rath SN. Soluble eggshell membrane: A natural protein to improve the properties of biomaterials used for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2016 Oct 1;67:807-821. doi: 10.1016/j.msec.2016.05.005. Epub 2016 May 5. PMID: 27287179.
- Choi HJ, Kim YM, Suh JY, Han JY. Beneficial effect on rapid skin wound healing through carboxylic acid-treated chicken eggshell membrane. Mater Sci Eng C Mater Biol Appl. 2021 Sep;128:112350. doi: 10.1016/j.msec.2021.112350. Epub 2021 Jul 30. PMID: 34474899.
- Ahlborn G, Sheldon BW. Identifying the components in eggshell membrane responsible for reducing the heat resistance of bacterial pathogens. J Food Prot. 2006 Apr;69(4):729-38. doi: 10.4315/0362-028x-69.4.729. PMID: 16629012.
- D'Ambrosio C, Arena S, Scaloni A, Guerrier L, Boschetti E, Mendieta ME, Citterio A, Righetti PG. Exploring the chicken egg white proteome with combinatorial peptide ligand libraries. J Proteome Res. 2008 Aug;7(8):3461-74. doi: 10.1021/pr800193y. Epub 2008 Jun 21. PMID: 18570458.
- Arias JL, Fernandez MS, Dennis JE, Caplan AI. The fabrication and collagenous substructure of the eggshell membrane in the isthmus of the hen oviduct. Matrix. 1991 Nov;11(5):313-20. doi: 10.1016/s0934-8832(11)80202-7. PMID: 1725804.
- Guru PS, Dash S. Sorption on eggshell waste--a review on ultrastructure, biomineralization and other applications. Adv Colloid Interface Sci. 2014 Jul;209:49-67. doi: 10.1016/j.cis.2013.12.013. Epub 2014 Jan 6. PMID: 24456801.
- Delwatta SL, Gunatilake M, Baumans V, Seneviratne MD, Dissanayaka MLB, Batagoda SS, Udagedara AH, Walpola PB. Reference values for selected hematological, biochemical and physiological parameters of Sprague-Dawley rats at the Animal House, Faculty of Medicine, University of Colombo, Sri Lanka. Animal Model Exp Med. 2018 Nov 21;1(4):250-254. doi: 10.1002/ame2.12041. PMID: 30891574; PMCID: PMC6388088.
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008 May 15;453(7193):314-21. doi: 10.1038/nature07039. PMID: 18480812.
- Elgharably H, Ganesh K, Dickerson J, Khanna S, Abas M, Ghatak PD, Dixit S, Bergdall V, Roy S, Sen CK. A modified collagen gel dressing promotes angiogenesis in a preclinical swine model of chronic ischemic wounds. Wound Repair Regen. 2014 Nov-Dec;22(6):720-9. doi: 10.1111/wrr.12229. Epub 2015 Jan 8. PMID: 25224310; PMCID: PMC4380279.
- Elgharably H, Roy S, Khanna S, Abas M, Dasghatak P, Das A, Mohammed K, Sen CK. A modified collagen gel enhances healing outcome in a preclinical swine model of excisional wounds. Wound Repair Regen. 2013 May-Jun;21(3):473-81. doi: 10.1111/wrr.12039. Epub 2013 Apr 22. PMID: 23607796; PMCID: PMC3685858.
- Khurana. Textbook Of Medical Physiology (2nd ed.). Elsevier. 2009; 180.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
