Archive of Urological Research
Biogenesis of Melia azedarach silver nanoparticles using leaves and fruits in breast and ovarian cancer cell lines
Kousalya Lavudi1#, Rekha Rani Kokkanti2#, Srinivas Patnaik1 and Josthna Penchalaneni2*
2Department of Biotechnology, Sri Padmavati Mahila VisvaVidyalayam, Tirupati, Andhra Pradesh, 517502, India
#These authors have contributed equally to this work
Cite this as
Lavudi K, Kokkanti RR, Patnaik S, Penchalaneni J (2023) Biogenesis of Melia azedarach silver nanoparticles using leaves and fruits in breast and ovarian cancer cell lines. Arch Urol Res 7(1): 008-016. DOI: 10.17352/aur.000044Copyright
© 2023 Lavudi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Folk medicine has been considered one of the novel remedies for treating cancers. Women's cancers are increasing worldwide, and disease recurrence has been a major threat all over the world. Our current study focused on the formation of silver nanoparticles (AgNPs) by organic methods and their chemo-preventive capacity against the breast (MCF-7) and ovarian cancer (PA-1) cell lines from humans by employing MTT, Flow cytometry, and migration assays. Plant extracts in organic nanoparticle production have become more common in recent years due to their benefits, including affordability, effectiveness, simplicity and briefness. Melia azedarach leaf and fruit methanolic extracts were used to successfully create silver nanoparticles simultaneously to evaluate the potency and efficacy of the extracts. Characterization studies were performed using synthesized M. azedarach silver nanoparticles (MA-AgNPs). A typical SPR peak was discovered ranging from 400 nm (leaf) and 427 nm (fruit) using absorption spectroscopy, with an average particle size of 92.5 nm (leaf) and 124.1 (fruit) nm. The zeta potential for Melia leaves and fruits was found to be -20.9 and -31.2 mV for the extracts. The relevant functional groups for the capping agent found in the extracts and silver nanoparticles formed as a result of the reduction of silver nitrate were identified using Fourier transform infrared spectroscopy (FTIR). Antimicrobial activity against Escherichia coli, Bacillus aureus, Staphylococcus aureus, and Klebsiella pneumonia would be useful in new antimicrobial medications being developed. MCF-7 and PA-1 cell lines were found to be more susceptible to the cytotoxic action of the biosynthesized nanoparticles. The silver nanoparticles that were synthesized exhibited extremely positive anti-cancer activity.
Introduction
The mortality and morbidity of cancer are a burden on the entire world. It is becoming more common and has a higher incidence globally. In all countries, it has emerged as a significant cause of death in the prime of life [1]. Breast and ovarian cancers are considered the highest diagnosed ones in women accounting for 15.5 % and 4.7 % of all cancer deaths in the world [2]. Genetics, viruses and lifestyle factors are the major factors among others resulting in uncontrolled proliferation of cancer cells [3].
Natural products that contain bioactive compounds are active resources for the treatment of cancer [4]. They may be derived from plants that have medicinal properties and a range of therapeutic effects; they also aim for numerous functioning proteins. Current anticancer medicines utilize natural products for about 75% of their chemical components [5]. As a result, testing the anticancer potential of various natural chemicals may be a useful method for creating new cancer-treating medications.
Since ancient times, the herb Melia azedarach L. has been employed as an herbal medicine [6]. M. azedarach is a kind of deciduous tree that is frequently grown in southern Korea [7]. Traditional medicine uses the leaves, fruits, bark, seed, and root and research has revealed that these parts of the plant have a variety of pharmacological effects, including those that are antiviral, anti-fertility, anthelmintic, antibacterial, hepatoprotective, antioxidant and cytotoxic in nature [8-13]. Terpenoids and limonoids (tetranortriterpenoid), which were discovered using GC-MS analysis, have the highest secondary metabolite concentrations in the plant components, followed by steroids and flavonoids [14,15]. Triterpenoids and steroids, as well as alkaloids and condensed tannins, were found in the MA ethanol extracts after phytochemistry analysis, which was conducted on both the seeds and the leaves [16,17]. Sadaf, et al. [18] confirmed the antitumor and cytotoxic effects of M. azedarach fruit extracts on MCF-7 cell lines. Ethyl acetate-derived M. azedarach wild-type leaves have shown a substantial antioxidant and bio-selective anti-cancer potential in T47D cell lines Ervina, et al. [19].
Naive M. azedarach leaf extract had previously been shown to have significant antibacterial and antioxidant activities in various cell lines [20]. M. azedarach-derived compounds have, at this point, been shown to have anticancer properties in numerous trials [21]. In in vivo models, Nerome, et al. [6] demonstrated that M. azedarach leaf extracts inhibited the tumor formation capacity and reduce the proliferative potential of several cancers. Using a breast cancer cell line as a test subject, M. azedarach methanolic extract was found to be highly cytotoxic [22].
Currently, the detection and treatment of new diseases heavily rely on the rapid growth of nanobiotechnology [23]. For their potential use in biomedical and bioengineering processes, biosynthesized AgNPs, a category of eco-friendly, economically viable, and biocompatible substances, have gained interest [24,25]. AgNPs, which exhibit broad spectrum and potent antibacterial characteristics, have been the focus over the past few years to combat antibiotic resistance in bacteria [26,27]. AgNPs have also been extensively explored as parts of cutting-edge anticancer drugs to better control cancer in the clinic, just like many other nanoparticles, such as gold, iron and Zinc [28,29]. In many cases, AgNPs are produced when reducing agents interact with silver ions. The field of nanotechnology has recently become interested in biological precursor-based synthesis, notably plant-based green synthesis.
In view of the above context, this research aims to elucidate the development of potential traditional herb-based antibacterial, antioxidant, and antiproliferative properties of silver nanoparticles (AgNPs) derived from a methanolic leaf and fruit extracts of Melia azedarach (MA-AgNPs) against MCF-7 and PA-1 cell lines. There are several reports supporting the anticancer properties of MA leaf, bark, fruit, and root extracts in the treatment of breast, colon, lung, hepatoma, liver, and prostate cancers [7,17,30-33]. A recent study by Shreshta, et al. [34] revealed that MA leaf extracts have a considerable amount of cytotoxic activity against human ovarian cancer cell lines. Melia azedarach L. extracts have not yet been thoroughly explored for their potential anticancer properties when used to treat ovarian cancer, as far as we are aware. Nonetheless, we can assume that this species' extract will exert a significant therapeutic potential due to synergistic effects to act as an anticancer agent. By demonstrating the impact of various biological activities of the aforementioned extracts, our research demonstrates the uniqueness of our work. The results of our research might offer more information on M. azedarach's possible health benefits in treating ovarian cancer.
Materials and methods
Plant material collection and preparation
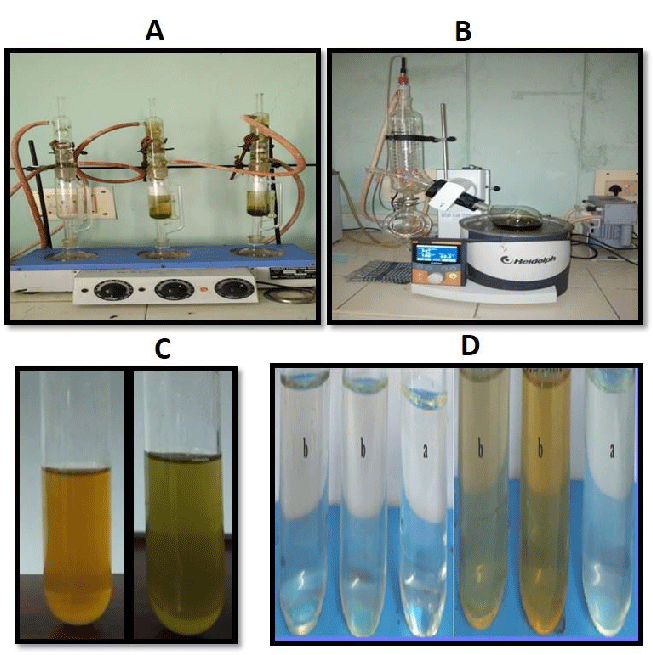
The plant material of Melia azedarach (MA) including leaves and fruits was collected from Salur, Vizianagaram district, Andhra Pradesh. The collected material was rinsed twice with running tap water followed by distilled water. Leaves from M.Azedarach were air dried for a week, Powder and the fruit pulp were collected from the respective leaves and fruits After preparation of fruit powder, 3 g of fruit powder was taken and mixed with 100 ml of distilled H2O in a water bath at 70°C for 30 minutes of incubation, later the sample was filtered by using Whatman no-1 filter paper. After the completion of filtering, the crude extract was collected and the sample was converted to green synthesis by using AgNO3. Using the powdered leaf extract, methanolic extraction was performed as mentioned in our previous studies [35]. The pure solution was kept in the rotary flash evaporator to evaporate methanol to obtain a crude extract of MA (Figure 1A-B).
Synthesis of silver nanoparticles
M. Azaderach leaf and fruit silver nanoparticles were synthesized as mentioned in our previous studies [36]. Reduction was confirmed by color change (Figure 1C and1D).
Characterization of biosynthesized leaf and fruit MA-AgNPs
The biosynthesized AgNPs of both leaf and fruit extracts were characterized with a 200 – 600 nm UV–visible spectrophotometer (NanoDrop 8,000 Spectrophotometer). X-ray Diffraction (XRD) measurements of AgNPs' crystalline structure were determined utilizing the XRD6000 (Shimadzu IR). Functional groups in MA-AgNPs were studied using FTIR (Fourier transform-infrared spectrum) Spectrophotometer with KBr pellets on a Shimadzu IR 8400 (Shimadzu IR). Using particle size and a zeta potential analyzer, the average particle size distribution, diameter, and stability were calculated (Horiba Nanopartica SZ-100 Nanoparticle analyzer). After the samples were filtered using 0.2 mm syringe filters, the clear nanoparticles solution was used for this study and measurements were taken at the proper range.
Antioxidant activity using DPPH
The stock solution was prepared by using 1mM of DPPH (2,2-diphenyl-1-picrylhydrazyl) solution in methanol [37]. 1 ml of stock solution was taken and 9 ml of methanol was added. 100 - 500 µg/ml of nano-synthesized sample was taken and methanol was added to bring the final volume to 1 ml. DPPH solution was added to each tube and kept for incubation for 30 min. O.D. values were taken at 517 nm, and the graph was plotted.
Anti-bacterial study
Plant extracts tend to exhibit antimicrobial activity by hindering the growth of pathogens surrounding them. Using the AgNP extracts by disc diffusion method microorganisms E. coli, B. subtilis, S. aureus, and K. pneumonia growth was measured on the nutrient agar gel. Melia azedarach fruit and leaf AgNP samples were treated on the microbial plates around the disk. After overnight incubation at 37 °C, the diameter at each treated concentration was used to calculate the zone of inhibition using a ruler from the bottom of the plate.
Cell viability analysis using MTT assay
MCF-7 and PA-1 cell lines were grown in 10% FBS-RPMI-1640 and Pen strep in a 60 mm dish. When cells are 80% confluent, 0.25% Trypsin-EDTA solution was used to dissociate the cells from the dish by incubating them in a CO2 chamber for 3 - 5 minutes. 10 ml of cultured media was added and under the microscope, the Neubauer chamber was used to count cells. A 96-well plate with 0.1 million seeded cells was treated with cells overnight. The next day, different concentrations of AgNP-derived methanolic plant extracts of both leaf and fruit were used for the treatment for 24 hours. As previously indicated, an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) experiment was conducted [38]. Readings were taken at 570nm using a spectrophotometer and the graph was plotted to calculate the respective inhibitory dose concentrations.
Apoptosis study using flow cytometer
1x 105 cells (PA1 and MCF-7) were cultured in a 6-well plate and incubated in a CO2 incubator overnight at 37 °C. A day later, cells were treated with both leaf and fruit MA-AgNPs for 24 hours. PBS was used to wash the cells and trypsinized and fixed them using chilled 70% ethanol and incubated for 30 minutes at 4 °C. Propidium Iodide (PI) staining was applied to suspended cells for 30 minutes at room temperature in the dark. The staining solution contained 0.05 ml RNase A and 0.45 ml PI. To assess cell cycle progression, Fluorescence-Activated Cell Sorting (FACS) Calibur flow cytometer was employed (Becton Dickinson, United States).
Cell migration analysis using wound healing assay
A migration experiment based on wound healing activity was carried out to assess the migration characteristics of MCF-7 cells. 6-well plates with a 1 * 106 density of cells were used. After 24 hours, the medium was changed once, and the process was performed once the cells had developed a confluent monolayer. A linear scratch was formed in every well using a 200 µl pipette tip. Culture media and leaf extracts of MA-AgNPs at various concentrations (10, 20 and 30 µg/ml) were added to wells after they had been washed with PBS. In the control cell sample, the scratch on the plate's bottom was examined at 200 x magnification at time points 0 and 24 hours after it had healed. The experiment was conducted with three biological replicates.
Statistical analysis
All the observations were carried out in triplets expressed as mean ± SEM. Analysis of variance (ANOVA) using Duncan’s multiple tests was applied to analyze the data using SPSS version 17.0 significant at P values less than 0.05.
Results and discussion
UV-Vis spectrophotometer
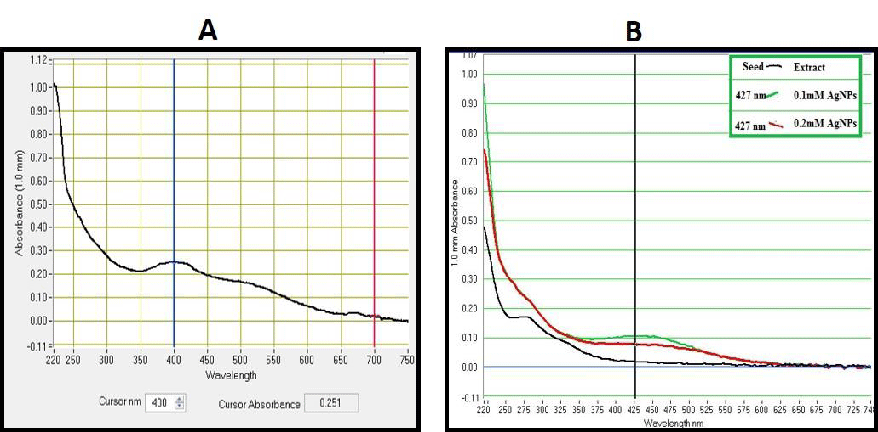
UV-Vis characterization has confirmed silver ion reduction to produce AgNPs and is observed by a color change (light yellow to dark brown). This reduction might happen due to the existence of a high number of small molecules in the colorless fruit extracts. After 10 minutes, the SPR spectra of biosynthesized leaf AgNPs were discovered at 427 nm (Figure 2A). Similar results were obtained using T. Stans leaf extracts [35]. Methanolic extracts of MA-AgNP fruit extracts have a wavelength of 427 nm (Figure 2B).
Particle size analyzer
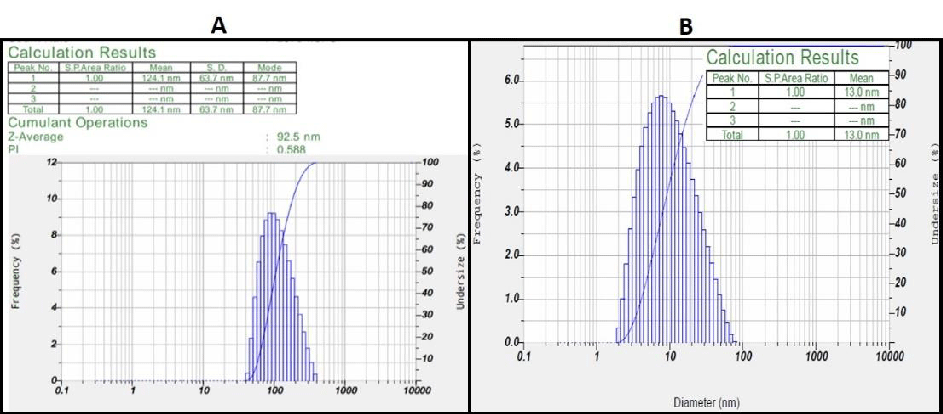
The poly-dispersed mixed solution's intensity and laser diffraction both contributed to the determination of the average particle size of the AgNPs. Methanolic leaf extracts have a size of 92.5 nm (Figure 3A), which is corresponded to the Macrotyloma seed AgNP extracts as reported by Lavudi, et al. [38]. However, the average diameter of the methanolic fruit AgNPs was observed to be 13.0 nm, with size distribution ranging in between 8-60nm. (Figure 3B) .
Zeta potential
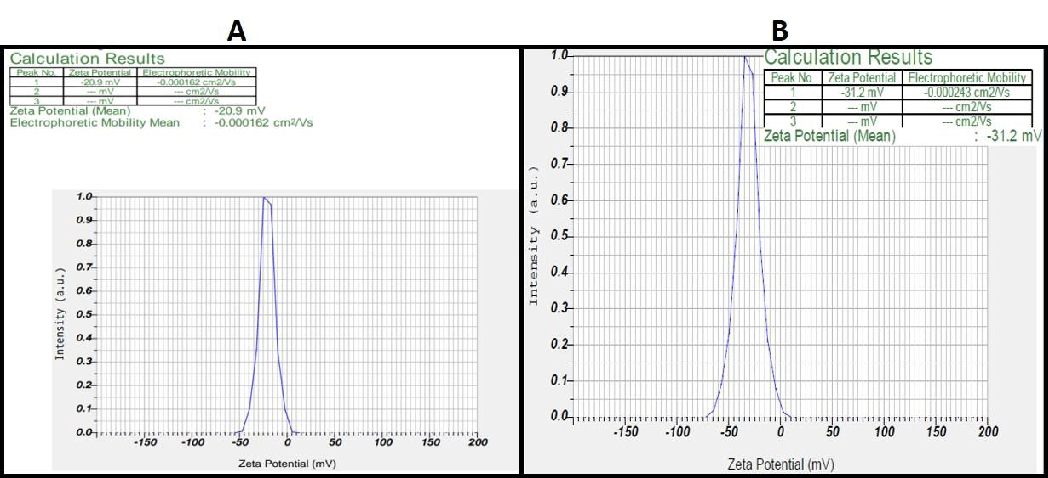
The stability of the synthesized AgNPs is considered a prime factor which is highly possible by forming negatively charged AgNPs which exhibit repulsion via electrostatic repulsive forces. The zeta potential for Melia leaf extracts was found to be -20.9 and -31.2 mV for the fruit extracts (Figure 4A-B). These negatively charged particles provide long-term stability and prevent agglomeration within the medium. Studies performed by Sandhya, et al. [36] have demonstrated a similar negative charge, proving that AgNP formed.
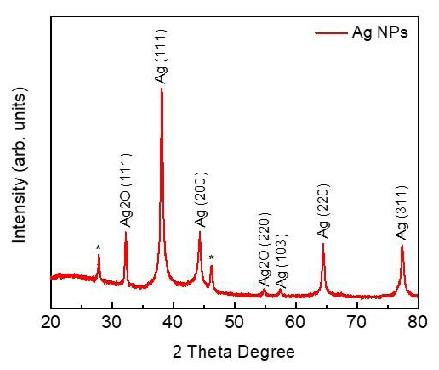
XRD
X-ray diffraction technique (XRD, D/Max 2005, Rigaku) was used to determine the crystal phase structure of synthesized MA leaf AgNPs. The (111), (200), (220), and (311) planes of the face-centered cubic (FCC) structure of metallic silver (JCPDS 04-0783) with Fm3m planetary group symmetry were assigned as the four significant and diversified peaks at 2 = 38.13, 44.36, 64.48, and 77.40 (Figure 5). Our results are quite significant with the work done by Chinnaswamy, et al. (2019). A small peak is also observed at 2θ = 57.46 (103) as FCC of metallic silver (JCPDS 41-1402) with P63/mmc group symmetry. Similarly, two distinct peaks of Ag2O are detected at 2θ = 32.7 and 54.9 corresponding to the silver oxide FCC planes (111) and (220) (JCPDS 43-0997) with Pn3m. Two peaks at 2θ = 27.80 and 46.19 has been marked with a star (*) that may correspond to silver or silver oxide. The size is determined to be between 22 and 35 nm for AgNPs when the Mean Crystallite Diameter (MCD) of as-synthesized nanomaterials is calculated using Scherrer's equation.
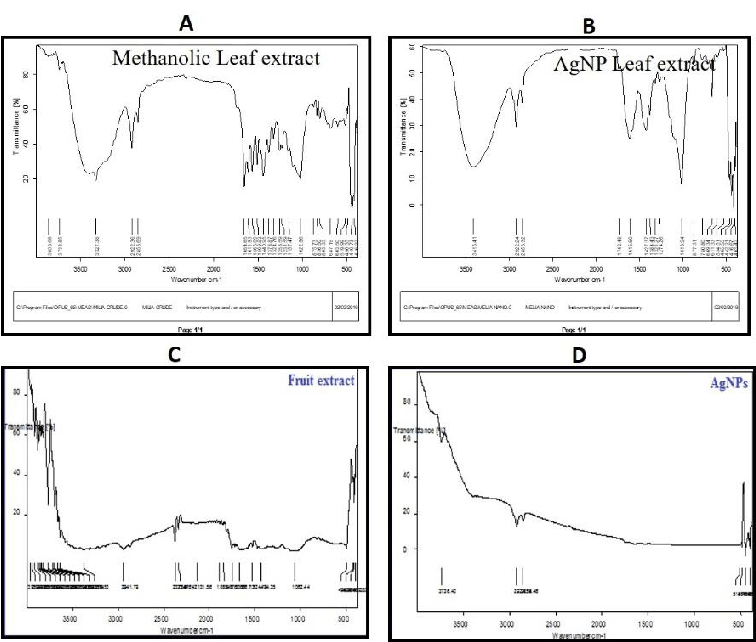
FTIR study
The leaves of MA-AgNPs FTIR spectra displayed notable peaks at 1031, 1370, 1614, 2923, and 3386 cm-1, which correlate to the existence of the C-C, C-O, C=N, C-H, O-H, and N-H stretches, respectively. The extract exhibited substantial peaks at 762.01, 821.37, 1030.80, 1204.84, 1336, 1447, 1534, 1611, 1701, 2920, 2851, 3353, and 3854 cm-1, which are indicative of the existence of C-O, C-N, C-C, OH and NH stretches with alcohols, alkanes and aromatic groups (Figure 6A-B). The FTIR spectrum of the silver nanoparticles made from fruit extracts is depicted in Figure 6C-D; it shows that the reduction of Ag+ to Ag occurs via interactions between the hydroxyl group and the stretch of the alkane H (O-H, 3332.25; C-H, 2896.91, 2834.33); and the carbonyl group and stretch of the alkenes groups (C=C, 2096.66; C=O, 1637.26 cm-1). It is possible to draw the conclusion that water-soluble essential oils are in charge of capping and effective stability.
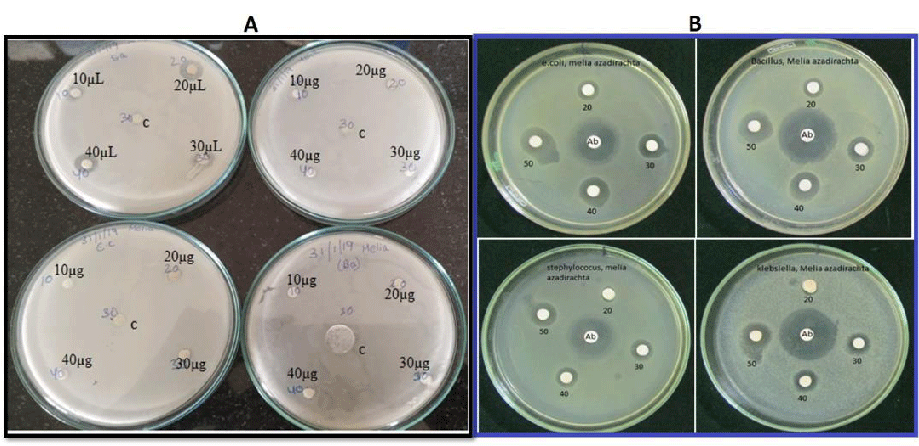
Anti-microbial activity
Inhibitory zones against the bacterial pathogens were observed after overnight incubation. the MA-Leaf AgNPs have exhibited bacterial resistance only against Staphylococcus aureus (Figure 7A). However, Melia fruit extracts have shown a significant amount of anti-bacterial activity against the 4 pathogens (Figure 7B). The antibacterial activity shown by the fruit extracts is probably due to the presence of limonoids which are present in fruit extracts [12,39].
Antioxidant activity
Green synthesis of leaf extract and fruit extract of this MA plant showed more antioxidant potential in comparison to the ascorbic acid standard using assay by the absorbance at 517 nm by UV-visible spectrophotometer. The average activity of green synthesized MA leaf and fruit extracts were 53.61 and 57 (Figure 8A-B).
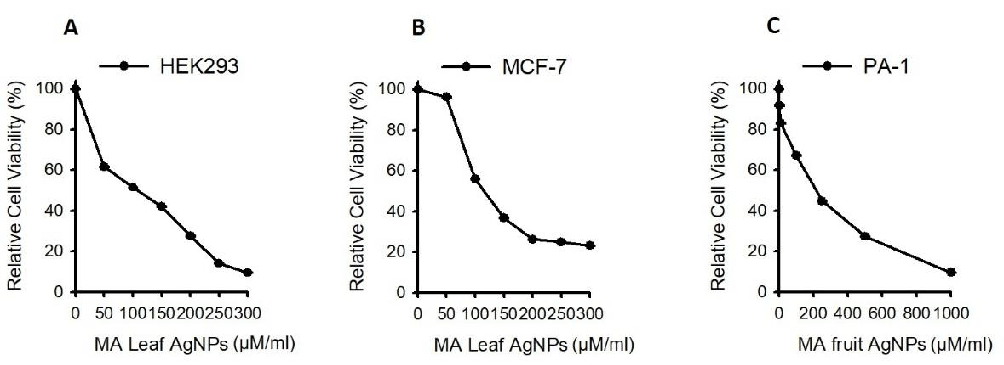
MTT assay
AgNP methanolic leaf and fruit extracts of MA were treated against both breast and ovarian cancer cell lines to study the cytotoxic effects. Samples were subjected to 24 hours of treatment with concentrations ranging from 0-250 µg/ml for 24 hours and MTT was performed and HEK293 was used as a control. Results depicted that methanolic MA-AgNP leaf extracts showed their efficacy and IC50 value was determined at 100 µg/ml concentrations (Figure 9A). Whereas using Methanolic AgNP fruit extracts on PA-1 IC50 was determined at 188.92 µg/ml (Figure 9B-C). Studies performed by Ashika, et al. [12] have shown that methanolic fruit extracts have shown anti-cancer activity against Breast, lung, stomach and leukemia cell line models.
Flow cytometry
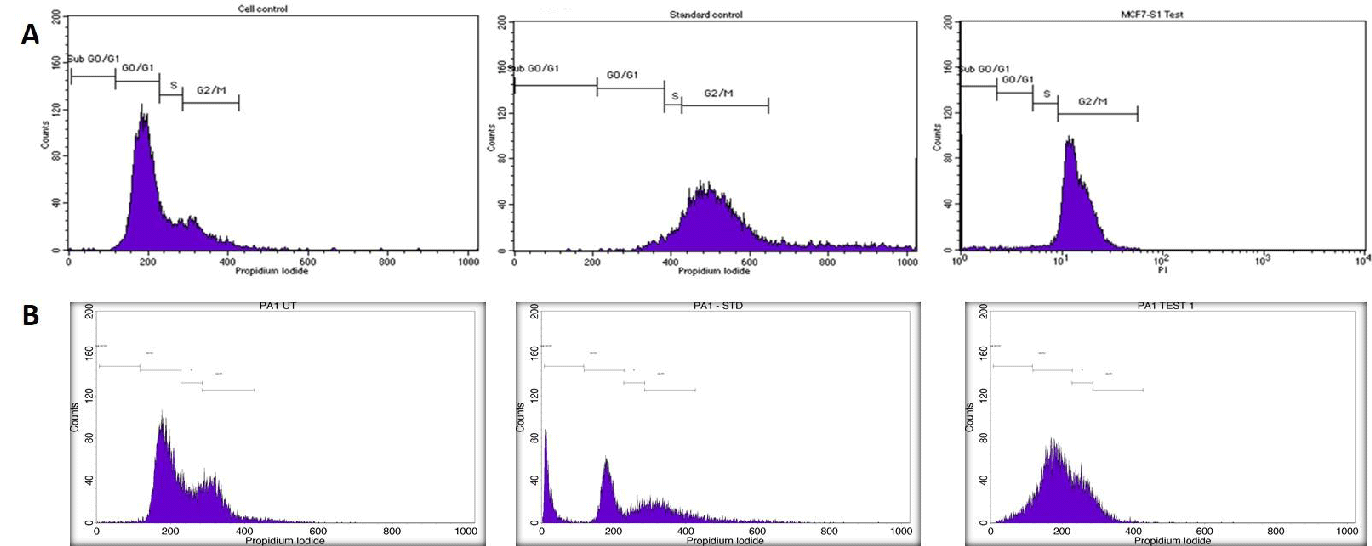
Utilizing this IC50 value as a benchmark, a flow cytometry investigation was carried out with both methanolic leaf and stem extracts in Breast and ovarian cancers. FACS analysis showed that on treating with Melia leaf extracts in MCF-7 cell lines, cell cycle arrest occurred in both G0/G1 and G1/s Phases. However, standard drug (Camptothecin, 5 µM) usage has shown the arrest at G0/G1 alone. However, fruit extracts when treated in the PA-1 ovarian cancer cell line, have shown an arrest at G2/M phases when treated with the Melia fruit AgNP extracts (Figure 10) . Our results are consistent with Wang, et al. [40] against leukemia cancer cell lines. Based on the cell cycle analysis, both leaf and fruit AgNP extracts have the ability to inhibit cell cycle progression and trigger apoptosis.
Migration assay
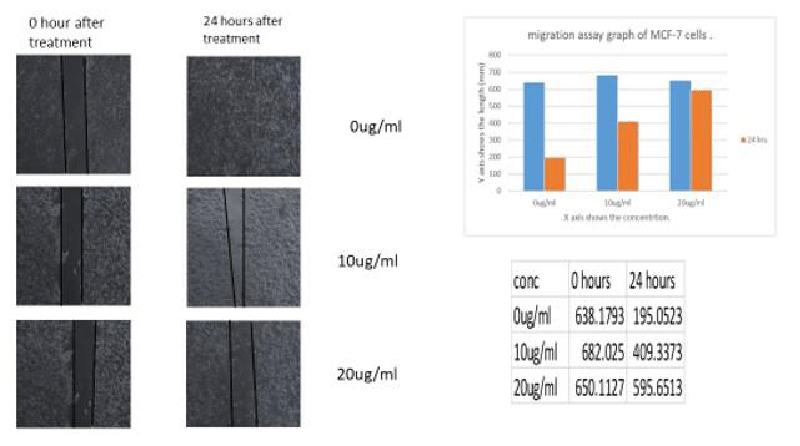
Migration capacity has been determined based on the analysis of wound healing. Migration capability decreased tremendously at 20 µg/ml when MCF-7 cells were treated with leaf methanolic AgNP extracts. Figure 11 shows the pictorial representation of the cells which have undergone migration and the comparisons were clear that methanolic MA-AgNP leaf extracts have the capacity to inhibit the rate of migration in breast cancer cell line MCF-7.
Conclusion
Our work demonstrated the anti-proliferative activity of a biosynthesized leaf and fruit extract of MA-AgNPs against breast and ovarian cancer cells in vitro. The evidence points to these extracts as promising anticancer agents that can be employed either alone or in combination with other chemotherapy medications to treat cancer. Future research should focus on a variety of issues by validating in vivo and in vitro to comprehend the molecular pathways involved in enhanced anti-cancer activity.
Kousalya Lavudi (KL): Methodology, writing original draft, review and analyze data
Rekha Rani Kokkanti (RRK): Writing an original draft, reviewing, editing, and proofreading
Srinivas Patnaik (SP): Supervision
Josthna Penchalaneni (PJ): Conceptualization
- Kemp JA, Kwon YJ. Cancer nanotechnology: current status and perspectives. Nano Converg. 2021 Nov 2;8(1):34. doi: 10.1186/s40580-021-00282-7. PMID: 34727233; PMCID: PMC8560887.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
- Fendereski A, Hajizadeh E, Haghighat S, Rasekhi A. Long-term outcomes of non-metastatic breast cancer patients by molecular subtypes. BMC Womens Health. 2022 Jul 4;22(1):268. doi: 10.1186/s12905-022-01846-3. PMID: 35787692; PMCID: PMC9254545.
- Della Bona A, Nedel F. Evaluation of Melia azedarach extracts against Streptococcus mutans. J Med Food. 2015 Feb;18(2):259-63. doi: 10.1089/jmf.2013.0181. Epub 2014 Jul 28. PMID: 25069066.
- Marimuthu S, Balakrishnan P, Nair S. Phytochemical investigation and radical scavenging activities of Melia azedarach and its DNA protective effect in cultured lymphocytes. Pharm Biol. 2013 Oct;51(10):1331-40. doi: 10.3109/13880209.2013.791323. Epub 2013 Jun 14. PMID: 23767787.
- Nerome K, Ito-Kureha T, Paganini T, Fukuda T, Igarashi Y, Ashitomi H, Ikematsu S, Yamamoto T. Potent and broad anticancer activities of leaf extracts from Melia azedarach L. of the subtropical Okinawa islands. Am J Cancer Res. 2020 Feb 1;10(2):581-594. PMID: 32195029; PMCID: PMC7061759.
- Jeba Malar TRJ, Antonyswamy J, Vijayaraghavan P, Ock Kim Y, Al-Ghamdi AA, Elshikh MS, Hatamleh AA, Al-Dosary MA, Na SW, Kim HJ. In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity. Saudi J Biol Sci. 2020 Feb;27(2):682-688. doi: 10.1016/j.sjbs.2019.11.024. Epub 2019 Nov 27. PMID: 32210688; PMCID: PMC6997857.
- de Sousa ALM, Rizaldo Pinheiro R, Furtado Araujo J, Mesquita Peixoto R, de Azevedo DAA, Cesar Lima AM, Marques Canuto K, Vasconcelos Ribeiro PR, de Queiroz Souza AS, Rocha Souza SC, de Amorim SL, Paula Amaral G, de Souza V, de Morais SM, Andrioli A, da Silva Teixeira MF. in vitro antiviral effect of ethanolic extracts from Azadirachta indica and Melia azedarach against goat lentivirus in colostrum and milk. Sci Rep. 2023 Mar 22;13(1):4677. doi: 10.1038/s41598-023-31455-5. PMID: 36949145; PMCID: PMC10031174.
- Hemdan BA, Mostafa A, Elbatanony MM, El-Feky AM, Paunova-Krasteva T, Stoitsova S, El-Liethy MA, El-Taweel GE, Abu Mraheil M. Bioactive Azadirachta indica and Melia azedarach leaves extracts with anti-SARS-CoV-2 and antibacterial activities. PLoS One. 2023 Mar 8;18(3):e0282729. doi: 10.1371/journal.pone.0282729. PMID: 36888689; PMCID: PMC9994683.
- Zahoor M, Ahmed M, Naz S, Ayaz M. Cytotoxic, antibacterial and antioxidant activities of extracts of the bark of Melia azedarach (China Berry). Nat Prod Res. 2015;29(12):1170-2. doi: 10.1080/14786419.2014.982649. Epub 2014 Nov 26. PMID: 25426766.
- Xiao J, Zhang Q, Gao YQ, Tang JJ, Zhang AL, Gao JM. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J Agric Food Chem. 2014 Apr 23;62(16):3584-90. doi: 10.1021/jf500054f. Epub 2014 Apr 9. PMID: 24689437.
- Akihisa T, Pan X, Nakamura Y, Kikuchi T, Takahashi N, Matsumoto M, Ogihara E, Fukatsu M, Koike K, Tokuda H. Limonoids from the fruits of Melia azedarach and their cytotoxic activities. Phytochemistry. 2013 May;89:59-70. doi: 10.1016/j.phytochem.2013.01.015. Epub 2013 Feb 27. PMID: 23465718.
- Sultana S, Asif HM, Akhtar N, Waqas M, Rehman SU. Comprehensive review on ethnobotanical uses, phytochemistry and pharmacological properties of Melia azedarach Linn. Asian J. Pharm. Res. Health Care. 2014; 6:26‑32.
- Efe B, Galata YF, Arslan YE. Assessment of the cytotoxicity of Melia azedarach L. extracts on human adipose-derived mesenchymal Stem Cells. 2018. https://doi.org/10.15671/HJBC.2018.220
- Sharma D, Paul Y. Preliminary and pharmacological profile of Melia azedarach L.: An overview. J. Appl. Pharm. Sci. 2013; 3:133‑8. https://doi.org/10.7324/JAPS.2013.31224
- Ervina M, Poerwono H, Widyowati R, Otsuka H, Matsunami K, Sukardiman S. Pregnane Steroids from the Leaves of Melia azedarach and Apoptotic Activity against T47D Cells. Asian Pac J Cancer Prev. 2021 Jun 1;22(6):1967-1973. doi: 10.31557/APJCP.2021.22.6.1967. PMID: 34181358; PMCID: PMC8418864.
- Ervina M, Sukardiman. A review: Melia azedarach L. as a potent anticancer drug. Pharmacogn. Rev. 2018; 12:94-102. https://doi.org/10.4103/phrev.phrev_41_17
- Mehreen Sadaf H, Bibi Y, Arshad M, Razzaq A, Ahmad S, Iriti M, Qayyum A. Analysis of Peganum harmala, Melia azedarach and Morus alba extracts against six lethal human cancer cells and oxidative stress along with chemical characterization through advance Fourier Transform and Nuclear Magnetic Resonance spectroscopic methods towards green chemotherapeutic agents. Saudi Pharm J. 2021 Jun;29(6):552-565. doi: 10.1016/j.jsps.2021.04.016. Epub 2021 Apr 23. PMID: 34194262; PMCID: PMC8233526.
- Ervina M, Poerwono H, Widyowati R, Matsunami K, Sukardiman. Bio-selective hormonal breast cancer cytotoxic and antioxidant potencies of Melia azedarach L. wild type leaves. Biotechnol Rep (Amst). 2020 Feb 17;25:e00437. doi: 10.1016/j.btre.2020.e00437. PMID: 32140442; PMCID: PMC7044715.
- Zhang L, Ismail MM, Rocchetti G, Fayek NM, Lucini L, Saber FR. The Untargeted Phytochemical Profile of Three Meliaceae Species Related to in vitro Cytotoxicity and Anti-Virulence Activity against MRSA Isolates. Molecules. 2022 Jan 10;27(2):435. doi: 10.3390/molecules27020435. PMID: 35056761; PMCID: PMC8777635.
- Chinnasamy G, Chandrasekharan S, Bhatnagar S. Biosynthesis of Silver Nanoparticles from Melia azedarach: Enhancement of Antibacterial, Wound Healing, Antidiabetic and Antioxidant Activities. Int J Nanomedicine. 2019 Dec 11;14:9823-9836. doi: 10.2147/IJN.S231340. PMID: 31849471; PMCID: PMC6913292.
- Jafari S, Saeidnia S, Hajimehdipoor H, Ardekani MR, Faramarzi MA, Hadjiakhoondi A, Khanavi M. Cytotoxic evaluation of Melia azedarach in comparison with, Azadirachta indica and its phytochemical investigation. Daru. 2013 May 16;21(1):37. doi: 10.1186/2008-2231-21-37. PMID: 23679992; PMCID: PMC3664079.
- Khatoon N, Mazumder JA, Sardar M. Biotechnological applications of green synthesized silver nanoparticles. J. Nanosci. Curr. Res. 2017; 2. https://doi.org/10.4172/2572-0813.1000107 4
- Ratan ZA, Haidere MF, Nurunnabi M, Shahriar SM, Ahammad AJS, Shim YY, Reaney MJT, Cho JY. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers (Basel). 2020 Apr 1;12(4):855. doi: 10.3390/cancers12040855. PMID: 32244822; PMCID: PMC7226404.
- Chaturvedi VK, Singh A, Singh VK, Singh MP. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr Drug Metab. 2019;20(6):416-429. doi: 10.2174/1389200219666180918111528. PMID: 30227814.
- Ahmad SA, Sabya SD, Ayesha K, Mohammed TA, Mohd A, Saquib HMD, Amit KN. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020; 3:756-769. https://doi.org/10.1016/j.mset.2020.09.002
- Slavin YN, Asnis J, Häfeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnology. 2017 Oct 3;15(1):65. doi: 10.1186/s12951-017-0308-z. PMID: 28974225; PMCID: PMC5627441.
- Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of Nanotechnology in Cancer Diagnosis and Therapy - A Mini-Review. Int J Med Sci. 2020 Oct 18;17(18):2964-2973. doi: 10.7150/ijms.49801. PMID: 33173417; PMCID: PMC7646098.
- Hanan NA, Chiu HI, Ramachandran MR, Tung WH, Mohamad Zain NN, Yahaya N, Lim V. Cytotoxicity of Plant-Mediated Synthesis of Metallic Nanoparticles: A Systematic Review. Int J Mol Sci. 2018 Jun 11;19(6):1725. doi: 10.3390/ijms19061725. PMID: 29891772; PMCID: PMC6032206.
- Kikuchi T, Pan X, Ishii K, Nakamura Y, Ogihara E, Koike K, Tanaka R, Akihisa T. Cytotoxic and apoptosis-inducing activities of 12-O-Acetylazedarachin B from the fruits of Melia azedarach in human cancer cell lines. Biol Pharm Bull. 2013;36(1):135-9. doi: 10.1248/bpb.b12-00795. PMID: 23302647.
- Kim HW, Kang SC. The Toxicity and Anti-cancer Activity of the Hexane Layer of Melia azedarach L. var. japonica Makino's Bark Extract. Toxicol Res. 2012 Mar;28(1):57-65. doi: 10.5487/TR.2012.28.1.057. PMID: 24278590; PMCID: PMC3834402.
- He Y, Wang J, Liu X, Zhang L, Yi G, Li C, He X, Wang P, Jiang H. Toosendanin inhibits hepatocellular carcinoma cells by inducing mitochondria-dependent apoptosis. Planta Med. 2010 Sep;76(13):1447-53. doi: 10.1055/s-0029-1240902. Epub 2010 Feb 15. PMID: 20157879.
- Ntalli NG, Cottiglia F, Bueno CA, Alché LE, Leonti M, Vargiu S, Bifulco E, Menkissoglu-Spiroudi U, Caboni P. Cytotoxic tirucallane triterpenoids from Melia azedarach fruits. Molecules. 2010 Aug 27;15(9):5866-77. doi: 10.3390/molecules15095866. PMID: 20802401; PMCID: PMC6257693.
- Shrestha SS, Ferrarese I, Sut S, Zengin G, Grana S, Ak G, Pant DR, Dall'Acqua S, Rajbhandary S. Phytochemical Investigations and in vitro Bioactivity Screening on Melia azedarach L. Leaves Extract from Nepal. Chem Biodivers. 2021 May;18(5):e2001070. doi: 10.1002/cbdv.202001070. Epub 2021 Mar 30. PMID: 33682999.
- Lavudi K, Harika GVS, Thirunavukarasou A, Govindarajan G, Srinivas S. Green Synthesis of Tecoma stans Flower and Leaf Extracts: Characterization and Anti-Proliferative Activity in Colorectal Cancer Cell Lines. Lett. Appl. NanoBioScience. 2022b. https://doi.org/10.33263/LIANBS123.061
- Sandhya PR, Penchalaneni J, Lavudi K, Gaddam SA, Kotakadi VS, Challagundala VN. Green synthesis of silver nanoparticles by leaf extracts of boerhavia erecta and spectral characterization and their antimicrobial, antioxidant ad cytotoxic studies on ovarian cancer cell lines. Lett. Appl. NanoBioScience. 2020; 9. 1165-1176 https://doi.org/10.33263/LIANBS93.11651176
- Oyedemi SO, Bradley G, Afolayan AJ. In-vitro and-vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr. J. Pharm. Pharmacol. 2010; 4:70-78. https://doi.org/10.5897/AJPP.9000176
- Lavudi K, Harika VS, Kokkanti RR, Patchigolla S, Sinha A, Patnaik S, Penchalaneni J. 2-Dimensional in vitro culture assessment of ovarian cancer cell line using cost effective silver nanoparticles from Macrotyloma uniflorum seed extracts. Front Bioeng Biotechnol. 2022 Aug 16;10:978846. doi: 10.3389/fbioe.2022.978846. PMID: 36051584; PMCID: PMC9425338.
- Shi YL, Li MF. Biological effects of toosendanin, a triterpenoid extracted from Chinese traditional medicine. Prog Neurobiol. 2007 May;82(1):1-10. doi: 10.1016/j.pneurobio.2007.02.002. Epub 2007 Feb 15. PMID: 17363132.
- Wang N, Fan Y, Yuan CM, Song J, Yao Y, Liu W, Gajendran B, Zacksenhaus E, Li Y, Liu J, Hao XJ, Ben-David Y. Selective ERK1/2 agonists isolated from Melia azedarach with potent anti-leukemic activity. BMC Cancer. 2019 Aug 2;19(1):764. doi: 10.1186/s12885-019-5914-8. PMID: 31375085; PMCID: PMC6679490.
- Pandey BP, Adhikari K, Pradhan S. In-vitro antioxidant, anti-cancer, and anti-inflammatory activities of selected medicinal plants from western Nepal. Futur. J. Pharm. Sci. 2020; 6:75. https://doi.org/10.1186/s43094-020-00107-0
- Tang XL, Yang XY, Kim YC, Kim SY, Kang BD, Choi DY, Kim OJ, Park WC, Park H. Protective effects of the ethanolic extract of Melia toosendan fruit against colon cancer. Indian J Biochem Biophys. 2012 Jun;49(3):173-81. PMID: 22803332.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
