Annals of Pancreatic Disorders and Treatment
Deciphering PPAR-inducing pathway clarifying the link between Alzheimer’s Disease and diabetes
Nesma S Shafie1, Yasser M Morsy2 and Marwa Amer1*
2Department of Gastroenterology and Hepatology, University Hospital Zurich and University of Zurich, Zurich, 8091, Switzerland
Cite this as
Shafie NS, Morsy YM, Amer M (2023) Deciphering PPAR-inducing pathway clarifying the link between Alzheimer’s Disease and diabetes. Ann Pancreat Disord Treatm 5(1): 001-005. DOI: 10.17352/apdt.000010Copyright
© 2023 Shafie NS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Diabetes Mellitus (DM)
Diabetes is a widely prevalent metabolic disorder characterized by chronic hyperglycemia. This condition is caused by various factors that ultimately result in pancreatic β cell failure. The American Diabetes Association (ADA) recognizes two main types of diabetes: Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM) [1-4]. In addition to Type 1 and Type 2 diabetes mellitus, there are two additional categories of Diabetes, namely Gestational Diabetes Mellitus (GDM) and monogenic diabetes.
The multisystemic nature of Diabetes implies that complications and comorbidities can impact multiple organ systems, especially critical organs such as the heart, brain, and kidneys, particularly when good glycemic control is not achieved [5]. Furthermore, sleep apnea has been identified as a comorbidity of type 2 diabetes, as it is more prevalent in individuals with diabetes than in those without diabetes [6,7]. Long-term complications of diabetes are primarily the result of vascular damage, which can be categorized as either macrovascular or microvascular. Microvascular complications include diabetic retinopathy, diabetic nephropathy (the leading cause of death in diabetic patients), and diabetic peripheral neuropathy, which remains a leading cause of blindness, end-stage kidney disease, and lower limb amputation [8]. Conversely, macrovascular complications are associated with an increased risk of coronary heart disease, peripheral vascular disease, cerebrovascular disease, and stroke [9]. In addition to the complications mentioned, Previous studies have suggested a strong association between Type 2 diabetes mellitus and late-onset Alzheimer’s Disease (AD) [10-13].
The correlation between AD and T2DM is primarily due to their shared pathophysiological characteristics [14,15]. While the exact mechanisms are not fully understood, several key proteins have been proposed as potential factors, which will be discussed in detail in this review. Insulin-dependent Type 1 diabetes mellitus (T1DM) is a condition that is prevalent among a minority of diabetics, accounting for only 5% - 10% [16]. The primary cause of T1DM is an autoimmune attack on the β cells [17].
Type 2 diabetes mellitus (T2DM) is an insulin-independent disorder that is prevalent among most diabetics, accounting for 90% - 95% of cases. The primary characteristic of T2DM is insulin resistance [18-21].
Alzheimer’s Disease (AD)
Alzheimer’s Disease (AD) is a neurodegenerative disorder that progresses gradually and is characterized by cognitive decline and memory loss [22,23]. Alzheimer’s Disease (AD) is the most prevalent form of dementia, accounting for 60% - 70% of all cases globally [24,25]. There is substantial evidence linking the pathogenesis of Alzheimer’s Disease (AD) to the deposition of amyloid-beta (Aβ) plaques and hyperphosphorylation of tau protein leading to neurofibrillary tangles [26-28].
However, the exact etiological factors/mechanisms and pathogenesis of AD remain uncertain [29,30]. While the accumulation of Aβ42 alone cannot fully account for the sequence of pathological events observed in Alzheimer’s Disease (AD), it can be attributed to dysregulated insulin/IGF-1 signaling [31-33].
Insulin resistance association with AD and DM
New findings indicate a close link between insulin resistance and Alzheimer’s Disease, similar to the case of chronic type 2 diabetes mellitus (T2DM) [31,34-37]. Epidemiological studies conducted over the past several decades have explored the correlation between changes in IR/IGF-R signaling pathways and other proteins associated with insulin signaling and Alzheimer’s Disease (AD). These studies suggest that there may be a relationship between these factors [7-13]. Despite extensive research, there are still unanswered questions regarding the interaction between T2DM and AD and how precisely T2DM influences the development of AD. Addressing these questions may provide valuable insights and guide the development of more effective therapeutic strategies.
Traditionally, the brain has been regarded as insulin-insensitive because insulin does not stimulate glucose metabolism in the brain [38,39]. It has been reported in recent studies that there are high densities of insulin receptors present in the brain [40]. According to these studies, the regulation of peripheral energy homeostasis is influenced by the hypothalamic action of insulin [38]. Insulin signaling has been shown to play a crucial role in various processes within the brain, including development, neuroprotection, metabolism, plasticity, and promotion of brain Aβ clearance. However, it also plays a significant role in neurodegeneration and neuropathological processes, such as cognitive decline and the development of Alzheimer’s Disease [40-42]. Therefore, the notion that Alzheimer’s Disease (AD) could be classified as type 3 diabetes has gained considerable support [43-46].
Alzheimer’s Disease, along with several other neurodegenerative diseases, displays neuro-inflammation as an early and consistent feature. [47,48]. The high expression of pro-inflammatory cytokines, including IL-1β and IL-6, provides further support for this association [47,49]. Consequently, these changes can lead to behavioral alterations, neuronal death, and accelerated disease progression. Furthermore, the increased expression of interferon-gamma and macrophage migration inhibitory factors in proximity to Aβ42 plaques can cause direct neuronal damage and death, as observed in the AD brain [47,48,50].
Peroxisome Proliferator-Activated Receptors (PPARs) participate in various molecular and enzymatic pathways that are DNA-dependent and independent in adipose tissue, liver, and skeletal muscles [51]. These pathways are disrupted in disease states and can lead to metabolic energy imbalances [52]. Hence, targeting PPARs can serve as a potential therapeutic strategy for a wide range of diseases, including but not limited to diabetes, obesity, inflammation, neurodegenerative disorders, and cancer [53-55]. PPARs have been found to play a role in regulating insulin-stimulated gene expression by sensing signals transmitted from cell surface membranes [56].
PPARs consist of three isoforms, which are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily [52,57,58]. The brain expresses all three isoforms of PPARs, with PPARβ/δ exclusively expressed in neurons and PPARα and γ expressed in both neurons and astrocytes [59]. PPARα is responsible for regulating energy homeostasis, while the activation of PPARγ leads to insulin sensitization and enhanced glucose metabolism. On the other hand, the activation of PPARβ/δ is associated with enhanced fatty acid metabolism. Therefore, the PPAR family is known to play a significant role in regulating energy homeostasis and metabolic function, in addition to its potent anti-inflammatory effects [56].
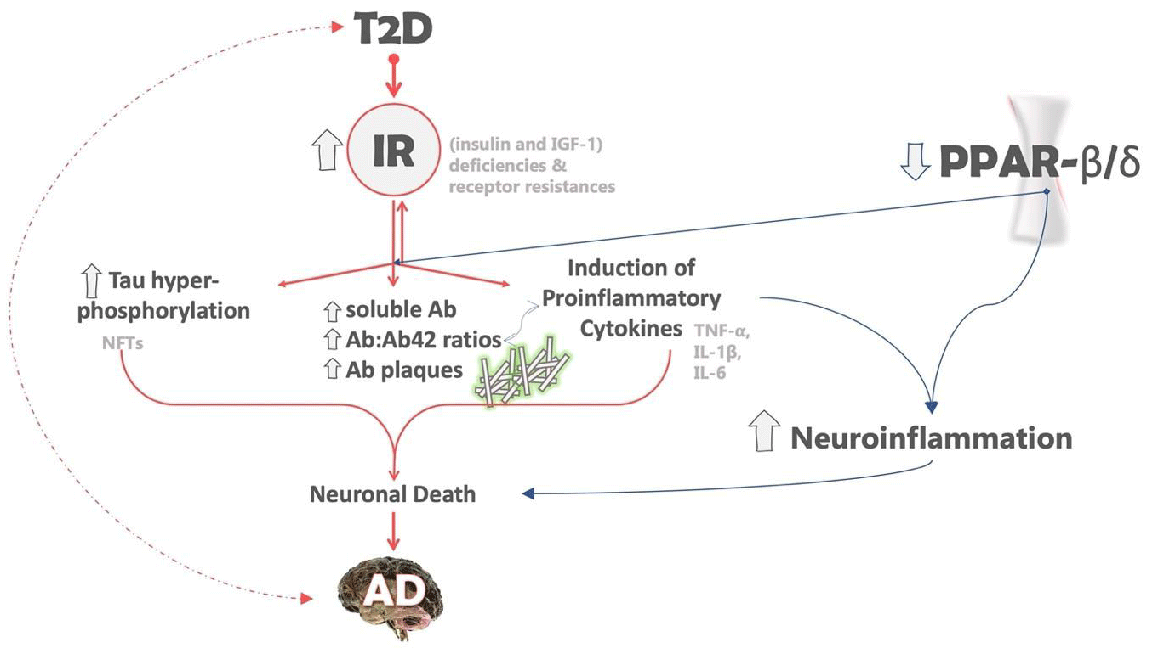
The downregulation of PPAR-β/δ in the brain is an AD-related abnormality that can be associated with both insulin resistance and neuroinflammation. PPAR-β/δ is highly expressed in the normal brain, like PPAR-α, while the gene expression of PPAR-γ is upregulated [56]. Moreover, experimental studies have shown that the depletion of PPAR- β/δ not only leads to an increase in neuroinflammation but also causes oxidative stress, astrogliosis and the deposition of Aβ42 and PHF tau in the brain [60] (Figure 1).
Insulin resistance (IR) in Type 2 diabetes (T2D) contributes to the formation of both Aβ plaques and tau hyperphosphorylation. Tau protein becomes hyperphosphorylated, detaches from microtubules, aggregates to neuronal fibrillary tangles. Additionally, the deposition of amyloid plaques triggers the release of proinflammatory responses such as IL-6, TNF-α, IFN-γ, and IL-α, leading to neuroinflammation and promoting neuronal injury. The combined effect of these factors leaves neurons vulnerable to various attacks, resulting in the gradual loss of synapses and eventually neuronal death. The accompanying figure illustrates the potential impact of PPAR-β/δ depletion on neuroinflammation and neurodegenerative disorders, including Alzheimer’s.
The dashed arrows suggest that the relationship between T2D and AD is likely bidirectional, although the precise mechanism is unclear. The arrows next to the gene names or biological processes indicate the effects of IR, with up-arrows indicating elevated/up-regulated genes or processes and down-arrows indicating down-regulated genes or processes. The different colors denote distinct roles in AD, with pink indicating the role of IR in AD and blue indicating the role of PPAR-β/δ in AD.
Awareness of the critical role of insulin in the brain and the insulin resistance observed in AD has led to attempts to overcome impaired insulin signaling and improve brain IR sensitivity. One approach is to use insulin sensitizers such as Peroxisome Proliferator-Activated Receptor (PPAR) agonists, which have shown promising early therapeutic effects on AD-associated molecular and biochemical brain pathologies [59,61, 62].
PPAR-β/δ agonist has been shown to effectively reduce astrocyte activation, providing an anti-inflammatory effect on glial cells. It also reduces neutrophil infiltration into the brain during ischemia and provides protection against neuroinflammation [56,63]. Studies have reported that PPAR-β/δ agonist has the ability to reduce amyloid burden, which includes Aβ42 deposition, and this effect may be mediated by its impact on amyloid clearance [56,64]. Aβ-oligomers can induce neuronal insulin resistance by promoting TNF-α secretion, leading to the activation of stress kinases IκB kinase (IKK) and double-stranded RNA-dependent protein kinase (PKR) [65,66].
This activation has been observed in AD animal models and can induce inflammation and endoplasmic reticulum stress, as well as deregulation of insulin signaling [28]. Together, these data suggest a feed-forward loop where Aβ oligomers amplify brain IR, which further decreases Aβ clearance and enhances the predisposition for Aβ oligomerization [67].
Collectively, the evidence supports the notion that insulin resistance plays a significant role in the pathogenesis of AD, leading to neuroinflammation and ultimately neurodegeneration. Early treatment with insulin sensitizers such as PPAR agonists, which possess both anti-inflammatory and neuroprotective properties, holds promise as a therapeutic strategy for mitigating AD and other neurodegenerative disorders.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019 Jan;42(Suppl 1):S13-S28. doi: 10.2337/dc19-S002. PMID: 30559228.
- Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020 Aug 30;21(17):6275. doi: 10.3390/ijms21176275. PMID: 32872570; PMCID: PMC7503727.
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017 Mar 30;3:17016. doi: 10.1038/nrdp.2017.16. PMID: 28358037.
- Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020 Nov;131:110708. doi: 10.1016/j.biopha.2020.110708. Epub 2020 Sep 11. PMID: 32927252.
- Mauricio D, Alonso N, Gratacòs M. Chronic Diabetes Complications: The Need to Move beyond Classical Concepts. Trends Endocrinol Metab. 2020 Apr;31(4):287-295. doi: 10.1016/j.tem.2020.01.007. Epub 2020 Feb 4. PMID: 32033865.
- Paschou SA, Bletsa E, Saltiki K, Kazakou P, Kantreva K, Katsaounou P, Rovina N, Trakada G, Bakakos P, Vlachopoulos CV, Psaltopoulou T. Sleep Apnea and Cardiovascular Risk in Patients with Prediabetes and Type 2 Diabetes. Nutrients. 2022 Nov 24;14(23):4989. doi: 10.3390/nu14234989. PMID: 36501019; PMCID: PMC9741445.
- Wilson NRC, Veatch OJ, Johnson SM. On the Relationship between Diabetes and Obstructive Sleep Apnea: Evolution and Epigenetics. Biomedicines. 2022 Mar 14;10(3):668. doi: 10.3390/biomedicines10030668. PMID: 35327470; PMCID: PMC8945691.
- Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018 Sep;61(9):1902-1912. doi: 10.1007/s00125-018-4692-1. Epub 2018 Jul 20. PMID: 30030554; PMCID: PMC6096638.
- Wei J, Tian J, Tang C, Fang X, Miao R, Wu H, Wang X, Tong X. The Influence of Different Types of Diabetes on Vascular Complications. J Diabetes Res. 2022 Feb 22;2022:3448618. doi: 10.1155/2022/3448618. PMID: 35242879; PMCID: PMC8888068.
- Hamzé R, Delangre E, Tolu S, Moreau M, Janel N, Bailbé D, Movassat J. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Shared Molecular Mechanisms and Potential Common Therapeutic Targets. Int J Mol Sci. 2022 Dec 4;23(23):15287. doi: 10.3390/ijms232315287. PMID: 36499613; PMCID: PMC9739879.
- Hobday AL, Parmar MS. The Link Between Diabetes Mellitus and Tau Hyperphosphorylation: Implications for Risk of Alzheimer’s Disease. Cureus. 2021 Sep 28;13(9):e18362. doi: 10.7759/cureus.18362. PMID: 34725612; PMCID: PMC8555851.
- Afzal M, Alharbi KS, Alzarea SI, Alyamani NM, Kazmi I, Güven E. Revealing genetic links of Type 2 diabetes that lead to the development of Alzheimer’s Disease. Heliyon. 2022 Dec 16;9(1):e12202. doi: 10.1016/j.heliyon.2022.e12202. PMID: 36711310; PMCID: PMC9876837.
- Moayedi K, Orandi S, Ebrahimi R, Tanhapour M, Moradi M, Abbastabar M, Golestani A. A novel approach to type 3 diabetes mechanism: The interplay between noncoding RNAs and insulin signaling pathway in Alzheimer’s Disease. J Cell Physiol. 2022 Jul;237(7):2838-2861. doi: 10.1002/jcp.30779. Epub 2022 May 17. PMID: 35580144.
- Janoutová J, Machaczka O, Zatloukalová A, Janout V. Is Alzheimer’s Disease a type 3 diabetes? A review. Cent Eur J Public Health. 2022 Sep;30(3):139-143. doi: 10.21101/cejph.a7238. PMID: 36239360.
- Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Ávila-Funes JA, Aguilar-Salinas CA. Pathophysiological Mechanisms Linking Type 2 Diabetes and Dementia: Review of Evidence from Clinical, Translational and Epidemiological Research. Curr Diabetes Rev. 2019;15(6):456-470. doi: 10.2174/1573399815666190129155654. PMID: 30648514.
- Ikegami H, Hiromine Y, Noso S. Insulin-dependent diabetes mellitus in older adults: Current status and future prospects. Geriatr Gerontol Int. 2022 Aug;22(8):549-553. doi: 10.1111/ggi.14414. Epub 2022 Jun 16. PMID: 35711119; PMCID: PMC9542793.
- Pang H, Luo S, Huang G, Xia Y, Xie Z, Zhou Z. Advances in Knowledge of Candidate Genes Acting at the Beta-Cell Level in the Pathogenesis of T1DM. Front Endocrinol (Lausanne). 2020 Mar 12;11:119. doi: 10.3389/fendo.2020.00119. PMID: 32226409; PMCID: PMC7080653.
- Saisho Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015 Feb 15;6(1):109-24. doi: 10.4239/wjd.v6.i1.109. PMID: 25685282; PMCID: PMC4317303.
- Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019 Jun;234(6):8152-8161. doi: 10.1002/jcp.27603. Epub 2018 Oct 14. PMID: 30317615.
- Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, Gundmi S, Jadhav R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2019 Mar;62(2):98-103. doi: 10.1016/j.rehab.2018.11.001. Epub 2018 Dec 13. PMID: 30553010.
- Wondmkun YT. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab Syndr Obes. 2020 Oct 9;13:3611-3616. doi: 10.2147/DMSO.S275898. PMID: 33116712; PMCID: PMC7553667.
- Weller J, Budson A. Current understanding of Alzheimer’s Disease diagnosis and treatment. F1000Res. 2018 Jul 31;7:F1000 Faculty Rev-1161. doi: 10.12688/f1000research.14506.1. PMID: 30135715; PMCID: PMC6073093.
- Zvěřová M. Clinical aspects of Alzheimer’s Disease. Clin Biochem. 2019 Oct;72:3-6. doi: 10.1016/j.clinbiochem.2019.04.015. Epub 2019 Apr 26. PMID: 31034802.
- Zhang Y, Gao H, Zheng W, Xu H. Current understanding of the interactions between metal ions and Apolipoprotein E in Alzheimer’s Disease. Neurobiol Dis. 2022 Oct 1;172:105824. doi: 10.1016/j.nbd.2022.105824. Epub 2022 Jul 22. PMID: 35878744.
- 2022 Alzheimer’s Disease facts and figures. Alzheimers Dement. 2022 Apr;18(4):700-789. doi: 10.1002/alz.12638. Epub 2022 Mar 14. PMID: 35289055.
- Novoa C, Salazar P, Cisternas P, Gherardelli C, Vera-Salazar R, Zolezzi JM, Inestrosa NC. Inflammation context in Alzheimer’s Disease, a relationship intricate to define. Biol Res. 2022 Dec 23;55(1):39. doi: 10.1186/s40659-022-00404-3. PMID: 36550479; PMCID: PMC9784299.
- Whitson HE, Colton C, El Khoury J, Gate D, Goate A, Heneka MT, Kaddurah-Daouk R, Klein RS, Shinohara ML, Sisodia S, Spudich SS, Stevens B, Tanzi R, Ting JP, Garden G; Symposium Planning Committee members. Infection and inflammation: New perspectives on Alzheimer’s Disease. Brain Behav Immun Health. 2022 Apr 22;22:100462. doi: 10.1016/j.bbih.2022.100462. PMID: 36118272; PMCID: PMC9475126.
- Roda AR, Serra-Mir G, Montoliu-Gaya L, Tiessler L, Villegas S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s Disease. Neural Regen Res. 2022 Aug;17(8):1666-1674. doi: 10.4103/1673-5374.332127. PMID: 35017413; PMCID: PMC8820696.
- Benmelouka AY, Ouerdane Y, Outani O, Alnasser YT, Alghamdi BS, Perveen A, Ashraf GM, Ebada MA. Alzheimer’s Disease-Related Psychosis: An Overview of Clinical Manifestations, Pathogenesis, and Current Treatment. Curr Alzheimer Res. 2022;19(4):285-301. doi: 10.2174/1567205019666220418151914. PMID: 35440308.
- Morgan SL, Naderi P, Koler K, Pita-Juarez Y, Prokopenko D, Vlachos IS, Tanzi RE, Bertram L, Hide WA. Most Pathways Can Be Related to the Pathogenesis of Alzheimer’s Disease. Front Aging Neurosci. 2022 Jun 24;14:846902. doi: 10.3389/fnagi.2022.846902. PMID: 35813951; PMCID: PMC9263183.
- Rad SK, Arya A, Karimian H, Madhavan P, Rizwan F, Koshy S, Prabhu G. Mechanism involved in insulin resistance via accumulation of β-amyloid and neurofibrillary tangles: link between type 2 diabetes and Alzheimer’s Disease. Drug Des Devel Ther. 2018 Nov 22;12:3999-4021. doi: 10.2147/DDDT.S173970. PMID: 30538427; PMCID: PMC6255119.
- Sharma VK, Singh TG. Insulin resistance and bioenergetic manifestations: Targets and approaches in Alzheimer’s Disease. Life Sci. 2020 Dec 1;262:118401. doi: 10.1016/j.lfs.2020.118401. Epub 2020 Sep 12. PMID: 32926928.
- Todri J, Lena O, Martínez Gil JL. An Experimental Pilot Study of Global Postural Reeducation Concerning the Cognitive Approach of Patients With Alzheimer’s Disease. Am J Alzheimers Dis Other Demen. 2020 Jan-Dec;35:1533317519867824. doi: 10.1177/1533317519867824. Epub 2019 Aug 8. PMID: 31394905.
- Sun Y, Ma C, Sun H, Wang H, Peng W, Zhou Z, Wang H, Pi C, Shi Y, He X. Metabolism: A Novel Shared Link between Diabetes Mellitus and Alzheimer’s Disease. J Diabetes Res. 2020 Jan 29;2020:4981814. doi: 10.1155/2020/4981814. PMID: 32083135; PMCID: PMC7011481.
- Kulkarni B, Kumar D, Cruz-Martins N, Sellamuthu S. Role of TREM2 in Alzheimer’s Disease: A Long Road Ahead. Mol Neurobiol. 2021 Oct;58(10):5239-5252. doi: 10.1007/s12035-021-02477-9. Epub 2021 Jul 18. PMID: 34275100.
- Kshirsagar V, Thingore C, Juvekar A. Insulin resistance: a connecting link between Alzheimer’s Disease and metabolic disorder. Metab Brain Dis. 2021 Jan;36(1):67-83. doi: 10.1007/s11011-020-00622-2. Epub 2020 Sep 28. PMID: 32986168.
- Meng L, Li XY, Shen L, Ji HF. Type 2 Diabetes Mellitus Drugs for Alzheimer’s Disease: Current Evidence and Therapeutic Opportunities. Trends Mol Med. 2020 Jun;26(6):597-614. doi: 10.1016/j.molmed.2020.02.002. Epub 2020 Mar 23. PMID: 32470386.
- De Felice FG, Benedict C. A Key Role of Insulin Receptors in Memory. Diabetes. 2015 Nov;64(11):3653-5. doi: 10.2337/dbi15-0011. PMID: 26494219.
- Milstein JL, Ferris HA. The brain as an insulin-sensitive metabolic organ. Mol Metab. 2021 Oct;52:101234. doi: 10.1016/j.molmet.2021.101234. Epub 2021 Apr 15. PMID: 33845179; PMCID: PMC8513144.
- Pomytkin I, Costa-Nunes JP, Kasatkin V, Veniaminova E, Demchenko A, Lyundup A, Lesch KP, Ponomarev ED, Strekalova T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci Ther. 2018 Sep;24(9):763-774. doi: 10.1111/cns.12866. Epub 2018 Apr 24. PMID: 29691988; PMCID: PMC6489906.
- Doust YV, Sumargo N, Ziebell JM, Premilovac D. Insulin Resistance in the Brain: Evidence Supporting a Role for Inflammation, Reactive Microglia, and the Impact of Biological Sex. Neuroendocrinology. 2022;112(11):1027-1038. doi: 10.1159/000524059. Epub 2022 Mar 11. PMID: 35279657.
- Tumminia A, Vinciguerra F, Parisi M, Frittitta L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int J Mol Sci. 2018 Oct 24;19(11):3306. doi: 10.3390/ijms19113306. PMID: 30355995; PMCID: PMC6275025.
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s Disease--is this type 3 diabetes? J Alzheimers Dis. 2005 Feb;7(1):63-80. doi: 10.3233/jad-2005-7107. PMID: 15750215.
- de la Monte SM, Wands JR. Alzheimer’s Disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008 Nov;2(6):1101-13. doi: 10.1177/193229680800200619. PMID: 19885299; PMCID: PMC2769828.
- Jang M, Choi N, Kim HN. Hyperglycemic Neurovasculature-On-A-Chip to Study the Effect of SIRT1-Targeted Therapy for the Type 3 Diabetes "Alzheimer’s Disease". Adv Sci (Weinh). 2022 Dec;9(34):e2201882. doi: 10.1002/advs.202201882. Epub 2022 Sep 8. PMID: 36073820; PMCID: PMC9731710.
- Michailidis M, Moraitou D, Tata DA, Kalinderi K, Papamitsou T, Papaliagkas V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int J Mol Sci. 2022 Feb 28;23(5):2687. doi: 10.3390/ijms23052687. PMID: 35269827; PMCID: PMC8910482.
- Rauf A, Badoni H, Abu-Izneid T, Olatunde A, Rahman MM, Painuli S, Semwal P, Wilairatana P, Mubarak MS. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules. 2022 May 17;27(10):3194. doi: 10.3390/molecules27103194. PMID: 35630670; PMCID: PMC9146652.
- Schain M, Kreisl WC. Neuroinflammation in Neurodegenerative Disorders-a Review. Curr Neurol Neurosci Rep. 2017 Mar;17(3):25. doi: 10.1007/s11910-017-0733-2. PMID: 28283959.
- Gabbouj S, Ryhänen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, Martiskainen H, Tanila H, Haapasalo A, Hiltunen M, Natunen T. Altered Insulin Signaling in Alzheimer’s Disease Brain - Special Emphasis on PI3K-Akt Pathway. Front Neurosci. 2019 Jun 18;13:629. doi: 10.3389/fnins.2019.00629. PMID: 31275108; PMCID: PMC6591470.
- Ogunmokun G, Dewanjee S, Chakraborty P, Valupadas C, Chaudhary A, Kolli V, Anand U, Vallamkondu J, Goel P, Paluru HPR, Gill KD, Reddy PH, De Feo V, Kandimalla R. The Potential Role of Cytokines and Growth Factors in the Pathogenesis of Alzheimer’s Disease. Cells. 2021 Oct 18;10(10):2790. doi: 10.3390/cells10102790. PMID: 34685770; PMCID: PMC8534363.
- Escandon P, Vasini B, Whelchel AE, Nicholas SE, Matlock HG, Ma JX, Karamichos D. The role of peroxisome proliferator-activated receptors in healthy and diseased eyes. Exp Eye Res. 2021 Jul;208:108617. doi: 10.1016/j.exer.2021.108617. Epub 2021 May 16. PMID: 34010603; PMCID: PMC8594540.
- Wagner N, Wagner KD. The Role of PPARs in Disease. Cells. 2020 Oct 28;9(11):2367. doi: 10.3390/cells9112367. PMID: 33126411; PMCID: PMC7692109.
- Qaoud MT, Almasri I, Önkol T. Peroxisome Proliferator-Activated Receptors as Superior Targets for Treating Diabetic Disease, Design Strategies - Review Article. Turk J Pharm Sci. 2022 Jun 27;19(3):353-370. doi: 10.4274/tjps.galenos.2021.70105. PMID: 35775494; PMCID: PMC9254082.
- Decara J, Rivera P, López-Gambero AJ, Serrano A, Pavón FJ, Baixeras E, Rodríguez de Fonseca F, Suárez J. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front Pharmacol. 2020 May 27;11:730. doi: 10.3389/fphar.2020.00730. PMID: 32536865; PMCID: PMC7266982.
- Sáez-Orellana F, Octave JN, Pierrot N. Alzheimer’s Disease, a Lipid Story: Involvement of Peroxisome Proliferator-Activated Receptor α. Cells. 2020 May 14;9(5):1215. doi: 10.3390/cells9051215. PMID: 32422896; PMCID: PMC7290654.
- Strosznajder AK, Wójtowicz S, Jeżyna MJ, Sun GY, Strosznajder JB. Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy. Neuromolecular Med. 2021 Mar;23(1):86-98. doi: 10.1007/s12017-020-08629-9. Epub 2020 Nov 18. PMID: 33210212; PMCID: PMC7929960.
- Gunasekaran V, Avarachan J, Augustine A, Khayum A, R A. 3-O-Acetyl-11-keto-β-boswellic acid ameliorates acquired, consolidated and recognitive memory deficits through the regulation of hippocampal PPAR γ, MMP9 and MMP2 genes in dementia model. Heliyon. 2021 Dec 2;7(12):e08523. doi: 10.1016/j.heliyon.2021.e08523. PMID: 34926858; PMCID: PMC8646985.
- Basilotta R, Lanza M, Casili G, Chisari G, Munao S, Colarossi L, Cucinotta L, Campolo M, Esposito E, Paterniti I. Potential Therapeutic Effects of PPAR Ligands in Glioblastoma. Cells. 2022 Feb 10;11(4):621. doi: 10.3390/cells11040621. PMID: 35203272; PMCID: PMC8869892.
- Reich D, Gallucci G, Tong M, de la Monte SM. Therapeutic Advantages of Dual Targeting of PPAR-δ and PPAR-γ in an Experimental Model of Sporadic Alzheimer’s Disease. J Parkinsons Dis Alzheimers Dis. 2018 May;5(1):10.13188/2376-922X.1000025. doi: 10.13188/2376-922X.1000025. Epub 2018 May 21. PMID: 30705969; PMCID: PMC6350901.
- Barroso E, del Valle J, Porquet D, Vieira Santos AM, Salvadó L, Rodríguez-Rodríguez R, Gutiérrez P, Anglada-Huguet M, Alberch J, Camins A, Palomer X, Pallàs M, Michalik L, Wahli W, Vázquez-Carrera M. Tau hyperphosphorylation and increased BACE1 and RAGE levels in the cortex of PPARβ/δ-null mice. Biochim Biophys Acta. 2013 Aug;1832(8):1241-8. doi: 10.1016/j.bbadis.2013.03.006. Epub 2013 Mar 16. PMID: 23507144.
- de la Monte SM. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs. 2017 Jan;77(1):47-65. doi: 10.1007/s40265-016-0674-0. PMID: 27988872; PMCID: PMC5575843.
- Govindarajulu M, Pinky PD, Bloemer J, Ghanei N, Suppiramaniam V, Amin R. Signaling Mechanisms of Selective PPARγ Modulators in Alzheimer’s Disease. PPAR Res. 2018 Oct 21;2018:2010675. doi: 10.1155/2018/2010675. PMID: 30420872; PMCID: PMC6215547.
- Chehaibi K, le Maire L, Bradoni S, Escola JC, Blanco-Vaca F, Slimane MN. Effect of PPAR-β/δ agonist GW0742 treatment in the acute phase response and blood-brain barrier permeability following brain injury. Transl Res. 2017 Apr;182:27-48. doi: 10.1016/j.trsl.2016.10.004. Epub 2016 Oct 18. PMID: 27818230.
- Amin AM, Mostafa H, Khojah HMJ. Insulin resistance in Alzheimer’s Disease: The genetics and metabolomics links. Clin Chim Acta. 2023 Jan 15;539:215-236. doi: 10.1016/j.cca.2022.12.016. Epub 2022 Dec 22. PMID: 36566957.
- Calabrò M, Rinaldi C, Santoro G, Crisafulli C. The biological pathways of Alzheimer disease: a review. AIMS Neurosci. 2020 Dec 16;8(1):86-132. doi: 10.3934/Neuroscience.2021005. PMID: 33490374; PMCID: PMC7815481.
- Burillo J, Marqués P, Jiménez B, González-Blanco C, Benito M, Guillén C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells. 2021 May 18;10(5):1236. doi: 10.3390/cells10051236. PMID: 34069890; PMCID: PMC8157600.
- Mullins RJ, Diehl TC, Chia CW, Kapogiannis D. Insulin Resistance as a Link between Amyloid-Beta and Tau Pathologies in Alzheimer’s Disease. Front Aging Neurosci. 2017 May 3;9:118. doi: 10.3389/fnagi.2017.00118. PMID: 28515688; PMCID: PMC5413582.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
