Global Journal of Infectious Diseases and Clinical Research
Mycoplasma pneumoniae-induced rash and mucositis: A rare extrapulmonary manifestation of Mycoplasma pneumoniae infection
Trilok Chand1*, Rushikesh Naik2 and Maya Banshidhar3
2Specialist Radiologist, Burjeel Hospital, Abu Dhabi, UAE
3General Practitioner Dentist, Tajmeel Dental Center, Abu Dhabi, UAE
Cite this as
Chand T, Naik R, Banshidhar M (2023) Mycoplasma pneumoniae-induced rash and mucositis: A rare extrapulmonary manifestation of Mycoplasma pneumoniae infection. Glob J Infect Dis Clin Res 9(1): 012-015. DOI: 10.17352/2455-5363.000056Copyright
© 2023 Chand T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Mycoplasma pneumoniae (M. pneumoniae) is a common atypical pathogen in humans, causing community-acquired pneumonia. Isolated Mycoplasma pneumoniae cases are commonly seen in the community, but an extrapulmonary manifestation like severe mucositis without skin lesions is rarely seen. The Mycoplasma-Induced Rash and Mucositis (MIRM) or Mycoplasma pneumoniae-Associated Mucositis (MPAM) is a severe manifestation of an atypical organism. Still, morbidity and mortality are less than Mycoplasma-Associated Steven Johnson’s Syndrome (MASJS) and Toxic Epidermal Necrolysis (TEN). We reported a case of a young adult admitted with pneumonia and rapidly developed severe mucositis without skin manifestations, which has increased his morbidity and recovery time.
Introduction
Infection of M. pneumoniae, a gram-negative bacteria, a member of the family Mycoplasmataceae, and order Mycoplasmatales, causes mycoplasma pneumonia [1]. M. pneumoniae commonly causes human disease and is one of the most common atypical pneumonia pathogens. Only 5% to 10% of people infected with M. pneumoniae develop pneumonia, mainly between 5 to 40 years of age [2]. M. pneumoniae also causes severe extrapulmonary diseases like haemolytic anaemia, oral and genital mucositis, conjunctivitis, and arthritis. Mucocutaneous eruptions are common extrapulmonary manifestations reported in approximately 25 percent of pediatric patients [3].
Canavan et al. performed a systematic review of 202 patients suffering from mucocutaneous lesions with positive M. pneumoniae testing and were the first to coin the term Mycoplasma pneumoniae-Induced Rash and Mucositis (MIRM) [4]. They also reported that MIRM correlated with a milder disease course, low rates of sequelae, and lower mortality than Erythema Multiforme (EM), Stevens-Johnson Syndrome (SJS), or Toxic Epidermal Necrolysis (TEN) [4,5]. SJS is a severe variant of the extrapulmonary manifestation of mycoplasma which has extensive blistering lesions, severe mucositis, and atypical target-like skin lesions which often progress to Toxic Epidermal Necrolysis (TEN) [6]. Typical mucositis in MIRM involves the oral cavity, orbit, and genitalia, and skin involvement is not necessary for all patients. The characteristic cutaneous lesions are vesiculobullous and/or atypical targetoid eruptions.
The two variants of MIRM coined were severe MIRM, which showed extensive involvement of atypical targetoid lesions or blisters, and MIRM sine rash, which showed minimal morbilliform lesions with few vesicles [4,5,7]. Interestingly, patients with MIRM sine rash had higher rates of mucosal involvement: oral (100%), ocular (92%), and urogenital (78%) [4,5].
Our case is a classic example of MIRM sine rash, who had mild respiratory symptoms of pneumonia, but he was crippled due to severe oral mucositis and odynophagia without skin lesions; however, he responded well with an antibiotic course and supportive treatment and was discharged safely.
Case history
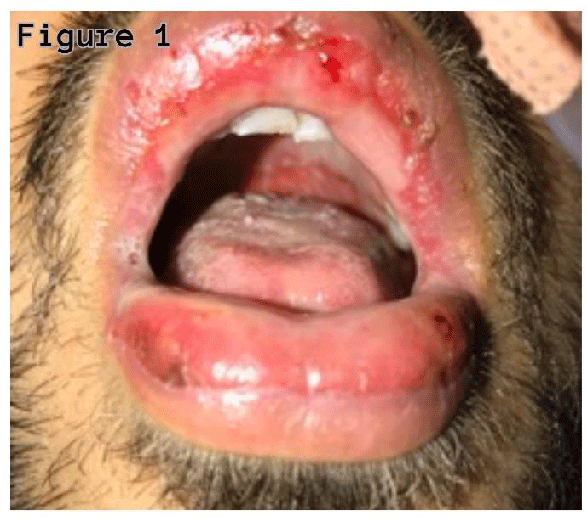
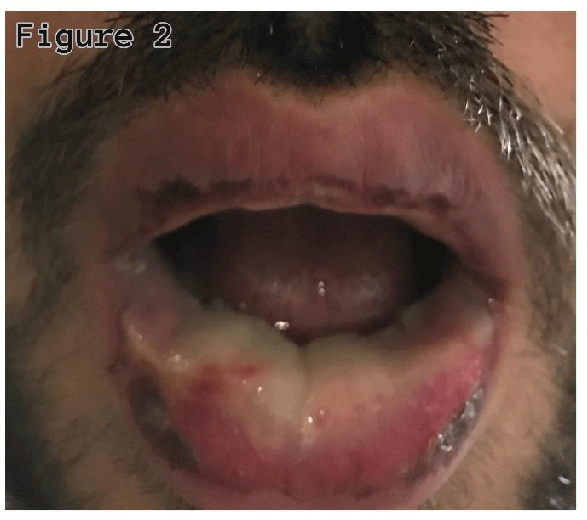
A twenty-seven-year-old non-smoker male was admitted with complaints of cough with sputum, fever, oral sores, redness in eyes, and odynophagia for four days. On examination, he was febrile (38.40’C), chest auscultation revealed bronchial breath sounds in the right axillary area, gross eye examination showed redness of the conjunctiva, and watering of both eyes, and oral inspection revealed widespread erythema, swelling, and sloughing of lips (Figure 1), palate and pharynx (Figure 2), however, there was no skin lesion observed.
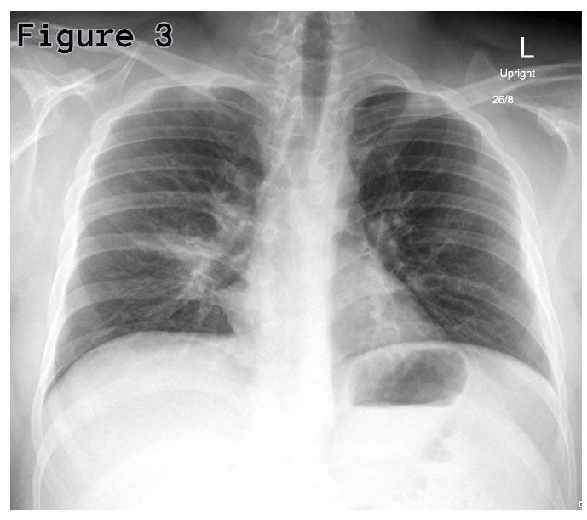
His routine blood investigations showed raised C-reactive protein (135.28 mg/L) and WBCs (12.51×103/mcL); however, his procalcitonin was normal (0.14 ng/mL). His Chest x-ray showed right lower lobe or middle lobe segmental consolidation (Figure 3). Patient’s sputum culture showed growth of normal flora; nevertheless, his serum mycoplasma IgM antibody was found positive, which is consistent with acute mycoplasma infection. His urine analysis showed red blood cell counts (RBCs) of 25-50 per high power field, suggestive of urethral mucositis.
He was started on intravenous Clarithromycin 500mg twice daily, intravenous fluid, pain relievers, oral analgesic gel, and nasogastric tube feeding. Gradually his condition improved, his oral mucositis started healing and his fever settled down. He was discharged on oral Clarithromycin after one week of intravenous therapy, and a total of 10 days of Clarithromycin course was given.
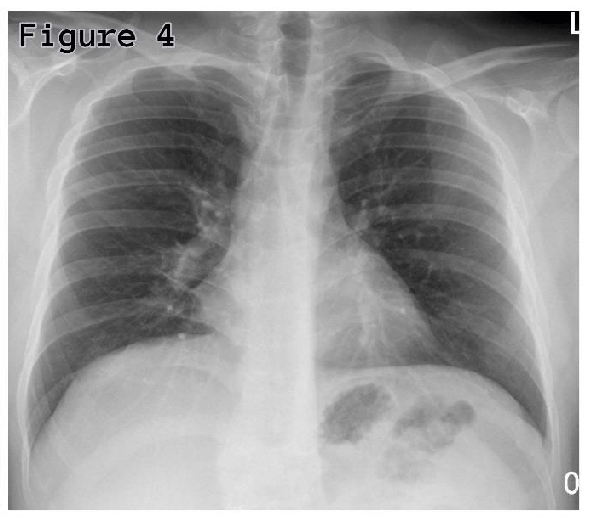
On two weeks follow-up, his chest x-ray showed significant resolution of right side consolidation (Figure 4) and there were no residual lesions in the oral cavity and urethra.
Discussion
The most common cause of atypical pneumonia in children and adults is M. pneumoniae, which is a ubiquitous organism and lacks a cell wall, hence does not take Gram’s staining. Around 7% - 20% of community-acquired pneumonia cases are due to M. pneumoniae infection [8-12]. The common respiratory symptoms of mycoplasma pneumonia are fever, malaise, dry cough, dyspnoea and occasional wheezing sounds; however, the symptoms are neither specific nor characteristic. The Japanese Respiratory Society (JRS) introduced six diagnostic criteria for Mycoplasma pneumonia: young age, absence of underlying disease, an intractable or non-productive cough, lack of crackles, and absence of leukocytosis [13]. The sensitivity and specificity of the JRS criteria were 88.6% and 69.8%, respectively, in patients aged < 60 years [14].
M. pneumoniae infection usually causes a mild respiratory system disease, but extrapulmonary manifestations of M. pneumoniae increase its severity and recovery time. M. pneumoniae also has severe extrapulmonary complications such as pericarditis, myocarditis, hepatitis, thrombocytopenic purpura, autoimmune hemolytic anaemia and neurologic manifestations, including acute cerebellar ataxia [15].
MIRM, previously known as MPAM, is an extrapulmonary manifestation of M. pneumoniae infection that has recently been distinguished from Erythema Multiforme (EM), Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) [4,16-18].
MIRM usually involves multiple mucosal sites, predominantly oral mucosa with vesiculobullous lesions and extensive oral erosions [19]. These patients have a less severe course of ocular and genital mucositis than oral mucositis [4]. In our case, the absence of recent drug hypersensitivity, typical or atypical target skin lesions, or extensive detachment of skin makes the diagnosis of EM, SJS and TEN unlikely.
The pathology of MIRM is not clearly understood; however, the most widely accepted theory suggests that cloning of B cells with cutaneous immune complex deposition and complement formation causes extrapulmonary manifestations [4]. Another possibility of MIRM pathology is molecular mimicry between Mycoplasma P1-adhesion molecules and a host’s keratinocytes [4].
MIRM diagnosis is based on clinical, radiological, and laboratory findings; however, there is no specific clinical and radiological feature. Diagnostic criteria for typical cases of MIRM proposed by Canavan et al. are summarized here [4].
Typical MIRM criteria
- < 10% BSA with detachment:
- mucosal sites involved
- Few vesiculobullous lesions or scattered atypical targets
- Presence or absence of targetoid lesions
- Atypical pneumonia:
- Signs: fever, cough, auscultatory or radiographic findings
- Labs: increase in M. pneumoniae IgM antibodies, M. pneumoniae in oropharyngeal or bullae cultures or PCR, and/or serial cold agglutinins.
The routine laboratory tests for diagnosis of MIRM include specimen culture, complement fixation, molecular-based detection assays such as PCR, and serologic testing [20].
The serological test may show raised IgM antibodies for M. pneumoniae, indicating a recent infection. For a more accurate diagnosis, paired serologic testing is required that demonstrates either seroconversion from the absence of IgM to the presence of IgM or a four-fold increase of IgG titers from acute to convalescent-phase samples [21]. The isolation of M. pneumoniae from a clinical specimen is difficult in routine clinical laboratories, as it requires a special culture media and also takes a more extended period for growth to have appeared in culture media. Other laboratory markers, such as CRP, WBCs, and procalcitonin used to be raised in bacterial infection, but not specific; however, in our case the procalcitonin level was normal.
Treatment is usually supportive with antibiotics, and specific therapy with immunosuppressive drugs or immunoglobulins did not show a better outcome in most studies and remains controversial [22]. Patients with mycoplasma pneumonia usually respond well to macrolide antibiotics (Azithromycin, Clarithromycin, and Erythromycin), doxycycline, and quinolones; however, resistance to macrolides is emerging, especially in Asia. The use of secondary antibiotics (tetracycline and fluoroquinolone), corticosteroids, or immunoglobulin has been considered as an alternative treatment for macrolide-resistant cases [23,24].
Early consultation with an infectious disease expert, ophthalmology, otolaryngology, dental, gastroenterology, and urology can help avoid long-term mucositis complications. The majority of MIRM cases recovered well without any long-term sequelae, like our case, which reported a full recovery in 2 weeks. Mortality in MIRM is low (3%), most patients fully recover from their disease, and recurrence is uncommon (8%) [5].
Conclusion
In general practice, a severe extrapulmonary manifestation of M. pneumoniae, such as MIRM, is not a common encounter; however, expediting a correct diagnosis can improve survival and avoid late complications or sequelae. The MIRM can be diagnosed by clinical signs and symptoms with positive serology for M. pneumoniae; however, culture in routine media is negative in most instances. Therefore, physicians should be aware of this entity and exclude other similar mucocutaneous manifestations like SJS and TEN. Our case is an unusual variant of MIRM which have no skin manifestations (MIRM sine rash).
Ethical consideration
Consent was obtained from the patient.
Approval from the internal ethical committee of our institute was taken for the publication of this case report.
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004 Oct;17(4):697-728, table of contents. doi: 10.1128/CMR.17.4.697-728.2004. PMID: 15489344; PMCID: PMC523564.
- Abdulhadi B, Kiel J. Mycoplasma Pneumonia. [Updated 2022 Jan 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430780/
- Gordon O, Oster Y, Michael-Gayego A, Marans RS, Averbuch D, Engelhard D, Moses AE, Nir-Paz R. The Clinical Presentation of Pediatric Mycoplasma pneumoniae Infections-A Single Center Cohort. Pediatr Infect Dis J. 2019 Jul;38(7):698-705. doi: 10.1097/INF.0000000000002291. PMID: 30985519.
- Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015 Feb;72(2):239-45. doi: 10.1016/j.jaad.2014.06.026. PMID: 25592340.
- Frantz GF, McAninch SA. Mycoplasma Mucositis. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525960/
- Kunimi Y, Hirata Y, Aihara M, Yamane Y, Ikezawa Z. Statistical analysis of Stevens-Johnson syndrome caused by Mycoplasma pneumonia infection in Japan. Allergol Int. 2011 Dec;60(4):525-32. doi: 10.2332/allergolint.11-OA-0309. PMID: 22113160.
- Li HO, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-Induced Rash and Mucositis With Cyclosporine [Formula: see text]. Journal of cutaneous medicine and surgery. 2019; 23(6): 608–612.
- Dumke R, Schnee C, Pletz MW, Rupp J, Jacobs E, Sachse K, Rohde G; Capnetz Study Group. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011-2012. Emerg Infect Dis. 2015 Mar;21(3):426-34. doi: 10.3201/eid2103.140927. PMID: 25693633; PMCID: PMC4344269.
- Pereyre S, Touati A, Petitjean-Lecherbonnier J, Charron A, Vabret A, Bébéar C. The increased incidence of Mycoplasma pneumoniae in France in 2011 was polyclonal, mainly involving M. pneumoniae type 1 strains. Clin Microbiol Infect. 2013 Apr;19(4):E212-7. doi: 10.1111/1469-0691.12107. Epub 2012 Dec 22. PMID: 23279613.
- Polkowska A, Harjunpää A, Toikkanen S, Lappalainen M, Vuento R, Vuorinen T, Kauppinen J, Flinck H, Lyytikäinen O. Increased incidence of Mycoplasma pneumoniae infection in Finland, 2010-2011. Euro Surveill. 2012 Feb 2;17(5):20072. doi: 10.2807/ese.17.05.20072-en. PMID: 22321135.
- Chalker V, Stocki T, Litt D, Bermingham A, Watson J, Fleming D, Harrison T. Increased detection of Mycoplasma pneumoniae infection in children in England and Wales, October 2011 to January 2012. Euro Surveill. 2012 Feb 9;17(6):20081. PMID: 22340973.
- Nir-Paz R, Abutbul A, Moses AE, Block C, Hidalgo-Grass C. Ongoing epidemic of Mycoplasma pneumoniae infection in Jerusalem, Israel, 2010 to 2012. Euro Surveill. 2012 Feb 23;17(8):20095. PMID: 22401504.
- Miyashita N, Matsushima T, Oka M, Japanese Respiratory Society. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med. 2006;45(7):419-28. doi: 10.2169/internalmedicine.45.1691. Epub 2006 May 1. PMID: 16679695.
- Ishida T, Hashimoto T, Arita M, Kaneshiro E, Osawa M, Tachibana H, Nishioka N, Watanabe K. Nihon Kokyuki Gakkai zasshi = the journal of the Japanese Respiratory Society. 2002; 40(12): 929–935.
- Narita M. Classification of Extrapulmonary Manifestations Due to Mycoplasma pneumoniae Infection on the Basis of Possible Pathogenesis. Front Microbiol. 2016 Jan 28;7:23. doi: 10.3389/fmicb.2016.00023. PMID: 26858701; PMCID: PMC4729911.
- Prindaville B, Newell BD, Nopper AJ, Horii KA. Mycoplasma pneumonia--associated mucocutaneous disease in children: dilemmas in classification. Pediatr Dermatol. 2014 Nov-Dec;31(6):670-5. doi: 10.1111/pde.12482. PMID: 25424207.
- Shah PR, Williams AM, Pihlblad MS, Nischal KK. Ophthalmic Manifestations of Mycoplasma-Induced Rash and Mucositis. Cornea. 2019 Oct;38(10):1305-1308. doi: 10.1097/ICO.0000000000001985. PMID: 31246679.
- Martínez-Pérez M, Imbernón-Moya A, Lobato-Berezo A, Churruca-Grijelmo M. Mycoplasma pneumoniae-Induced Mucocutaneous Rash: A New Syndrome Distinct from Erythema Multiforme? Report of a New Case and Review of the Literature. Actas Dermosifiliogr. 2016 Sep;107(7):e47-51. English, Spanish. doi: 10.1016/j.ad.2015.09.023. Epub 2016 Mar 31. PMID: 27040303.
- Morand M, Hatami A. Multiple superficial oral mucoceles after Mycoplasma-induced mucositis. Pediatr Dermatol. 2018 Jul;35(4):e210-e211. doi: 10.1111/pde.13515. Epub 2018 May 15. PMID: 29766572.
- Thurman KA, Walter ND, Schwartz SB, Mitchell SL, Dillon MT, Baughman AL, Deutscher M, Fulton JP, Tongren JE, Hicks LA, Winchell JM. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis. 2009 May 1;48(9):1244-9. doi: 10.1086/597775. PMID: 19331586.
- Lee WJ, Huang EY, Tsai CM, Kuo KC, Huang YC, Hsieh KS, Niu CK, Yu HR. Role of Serum Mycoplasma pneumoniae IgA, IgM, and IgG in the Diagnosis of Mycoplasma pneumoniae-Related Pneumonia in School-Age Children and Adolescents. Clin Vaccine Immunol. 2017 Jan 5;24(1):e00471-16. doi: 10.1128/CVI.00471-16. PMID: 27760779; PMCID: PMC5216438.
- Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Critical care medicine. 2011; 39(6): 1521-1532.
- Yang HJ, Song DJ, Shim JY. Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2017 Jun;60(6):167-174. doi: 10.3345/kjp.2017.60.6.167. Epub 2017 Jun 22. PMID: 28690643; PMCID: PMC5500384.
- Youn YS, Lee SC, Rhim JW, Shin MS, Kang JH, Lee KY. Early Additional Immune-Modulators for Mycoplasma pneumoniae Pneumonia in Children: An Observation Study. Infect Chemother. 2014 Dec;46(4):239-47. doi: 10.3947/ic.2014.46.4.239. Epub 2014 Dec 29. PMID: 25566403; PMCID: PMC4285006.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
