International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Nanozyme catalytic mimetic effect of iron oxide nanoparticles hybrids with cellulosic matrices and its synergism with microorganisms
Leonardo Zanata and Derval dos Santos Rosa*
Cite this as
Zanata L, Santos Rosa DD (2023) Nanozyme catalytic mimetic effect of iron oxide nanoparticles hybrids with cellulosic matrices and its synergism with microorganisms. Int J Nanomater Nanotechnol Nanomed 9(1): 001-003. DOI: 10.17352/2455-3492.000049Copyright License
© 2023 Zanata L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Iron Oxide Nanoparticles (IONPs) are generally assumed to be biologically inert, presenting chemical stability and low toxicity, and they can be hybridized with cellulosic matrixes aiming for biological applications (e.g. nanozymes). Two hydrothermal coprecipitation methods were applied, aiming to produce 2 different size Iron oxide nanoclusters, using ferric chloride and ferrous chloride, as well as nitrocellulose and cellulosic residues for the hybrids. The obtained materials were tested for catalytic effect in comparison and in synergy with catalase-positive P. aeruginosa, S. aureus, and B. subtilis bacterial strains. The catalytic effect was observed for all obtained materials and microorganisms, Due to the bivalent and trivalent iron molecules distributed along IONP cubic crystalline inverse spinel structures. Michaelis-Menten constant (Km) of IONP-and hybrids was higher in synergy with S. aureus in comparison with the results obtained by the microorganism alone, for instance, the best enzymatic efficiency for O2 release from hydrogen peroxide among the tested microorganisms. However, no significant difference was observed for most of the obtained materials alone. On the other hand, IONPs may help microorganisms as mimetic catalytic enzymes, when applied in synergy whit them.
Introduction
Iron Oxide Nanoparticles (IONPs) have unique properties such as Superparamagnetic behavior (SPM) at room temperature, chemical stability, low toxicity, and formation of magnetic monodomains [1,2]. IONPs can also be hybridized with cellulosic matrixes, as the backbone or compatibilization structures, for biological applications. They are generally assumed to be biologically inert and have been described as having enzyme-like activities and cofactors in metabolic processes [3-6], so they’ve also been called nanozymes [7,8]. Catalytic activities for both enzymes and nanozymes may be characterized by the degradation of hydrogen peroxide in oxygen and water [8,9]. To form nano magnetite (Fe3O4) as main IONPs by coprecipitation, during hydrothermal synthesis in a strongly alkaline medium, Parameters such as temperature, agitation speed, and concentration of the alkaline medium influence the reaction speed, and consequently, the size of the nanocrystals, thus generating nanoclusters with different physicochemical properties, such as hybrid dimensions and different mimic catalytic responses.
Methodology
For this work, two coprecipitation methods were applied. In method "A" [1,10,11], a suspension containing 4.0 g of FeCl2.4H2O and 8.0 g of FeCl3.6H2O in 150 mL of distilled water. This mixture was heated to 60 °C under magnetic stirring, and the medium was energized with N2. 60 ml of 3 M NaOH was added to the mixture slowly and dropwise while the temperature was maintained at 45 °C until the pH of the medium reached 12. The resulting particles were magnetically separated and washed repeatedly with deionized water until the pH of the medium was neutral.
For method “B”, the methodology has been repeated, but with the following modifications: The mixture was not heated, receiving agitation at 10,000 rpm by ultraturrax. 20 ml 8 M NaOH was quickly added to the mixture, raising the pH of the medium to 12, followed by cooling in an ice bath (up to a temperature of 25 °C) to stop co-precipitation. Microcrystalline Cellulose (MC) and Lignocellulosic Residues (LR) were used as cellulosic matrices to produce the hybrid in a mass reason of 2:1 for the ferric materials, being added to the coprecipitation mixture during the inertization phase.
For the catalytic mimetic effect, a modified catalase [6] test was applied. A 288 ml BOD flask was filled whit a 1 M H2O2 solution. Then 5 ml L-1 of 3 catalase-positive microorganisms’ suspension was tested (Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis) after 48h inoculation in nutrient broth, followed by a 109 CFU adjustment. Each of the 6 obtained nanohybrids (IONP-A, IONP-B; IONP+MC-A, IONP+MC-B, IONP+LR-A, IONP+LR-B) were also applied, in the concentration of 10 g L-1 for catalytic activity.
Dissolved oxygen was monitored by an oximeter (Hanna HI-9146, USA), under stirring for~120 RPM, for 1 hour and readings were taken every 5 minutes. The given materials were characterized elsewhere [12], to determine their size, properties, and iron oxide types produced, as well as how the IONPs bind to the cellulosic backbone matrixes. Tests were carried out in triplicate, and ANOVA was applied as a statistic tool, considering p > 0,05 for significant differences. Graphs and mathematic modeling were made using Origin Software.
Results
The results of the average oxygen release in the aqueous medium produced by the microorganisms, IONPs and its synergic effect are shown in Figures 1 to 4.
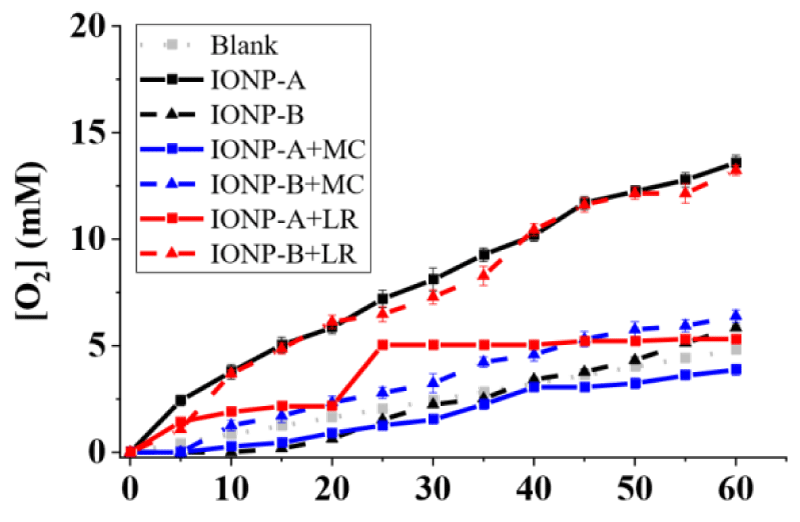
Catalytic effects (e.g., O2 release as dissolved oxygen) were observed for all hybrids (Figure 1). IONP-A and IONP+LR-B presented the best performance among the IONPs and hybrids, reaching 13,59 ± 0,35 and 13,23 ± 0,24 mM O2 after 1h, showing no significant difference between them.
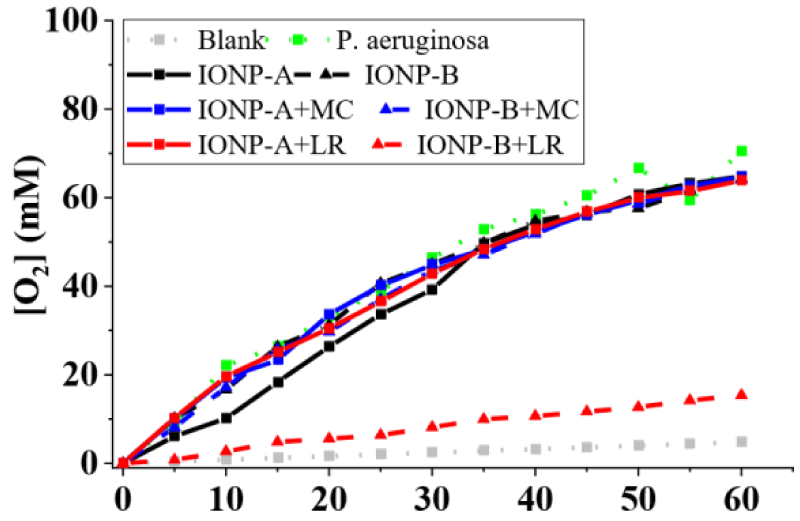
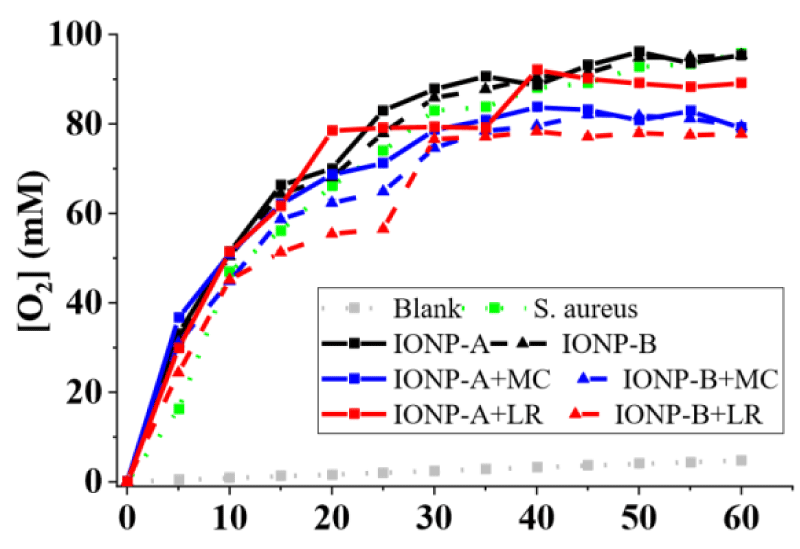
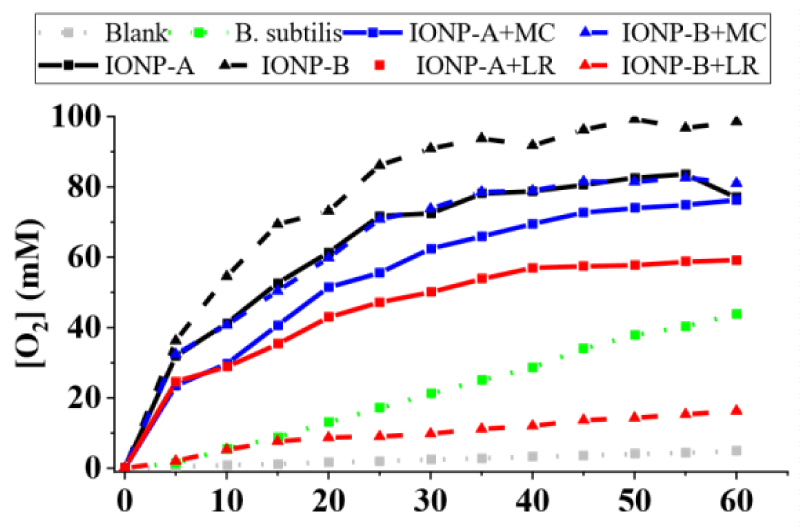
On the other hand, microorganisms’ catalytic effect for P. aeruginosa (Figure 2), S. aureus (Figure 3) and B. subtilis (Figure 4) showed higher releases than the IONPs, reaching 70,56 ± 0,52; 95,76 ± 0,47 and 43,92 ± 0,54 mM O2 in 1h, respectively. Synergic/antagonic effects of IONPs, IONPs, and cellulosic matrixes hybrids with microorganisms may also be observed. There was no significant difference between P. aeruginosa (Figure 4) alone and in the presence of the majority of the IONPs and hybrids, otherwise for IONP+LR-B, which presented an antagonic effect. For S. aureus (Figure 3) there was little interference in its catalytic action for IONPs A and B. However, IONP+LR-A helped the microorganisms, as discussed below. We also clarify that synergic and additive effects were treated as synonyms since we cannot separate one effect from the other. Still, for B. subtilis (Figure 4), a synergic effect was observed for the majority of the IONPs and hybrids, otherwise for IONP+LR-B, which presented an antagonic effect. IONPs catalytic effect is given by the bivalent and trivalent iron molecules distributed along its cubic crystalline inverse spinel structures [7,8,13]. Since there are plenty of those species in the produced IONPS and its hybrids [12,14], the catalytic effect was expected. However, IONPs-B shall present nanoclusters whit higher packaging, due to its obtaining process, leading to lower exposure of its iron atoms, thus having a lower catalytic effect. It’s also important to highlight that the chosen microorganisms present different types and amounts of extracellular enzymes, which leads to different results, due to this nonhomogeneous enzymatic mechanism among them.
On the other hand, comparing the Michaelis-Menten enzymatic model and the Michaelis-Menten constant (Km) of IONP-A and IONP+LR-B whit the substrate, it was 91,2 and 117,9 respectively, far higher than those achieved by S. aureus alone (19,6) and in synergy with IONP+LR-A (10,9), as well IONP+MC+A (8,1). S. aureus also presented the best enzymatic efficiency for O2 release from hydrogen peroxide among the tested microorganisms, since as lower the MichaelisMenten constant (Km) of an enzyme substrate, the lower shall be the concentration of the substrate to bind to the enzyme and it catalyzes the reaction [4,13,15,16].
Conclusion
Since the main purpose of this work was to evaluate the influence of the two coprecipitated IONP catalytic properties, in comparison with catalase microorganisms, it was possible to verify that all the obtained materials in fact preset catalytic capacity, however, no significant difference were observed for most of them. On the other hand, IONPs may help microorganisms as mimetic catalytic nanozymes, when applied in synergy whit them.
- Zarei S, Niad M, Raanaei H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J Hazard Mater. 2018 Feb 15;344:258-273. doi: 10.1016/j.jhazmat.2017.10.009. Epub 2017 Oct 12. PMID: 29055199.
- Dheyab MA, Aziz AA, Jameel MS, Noqta OA, Khaniabadi PM, Mehrdel B. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Sci Rep. 2020 Jul 1;10(1):10793. doi: 10.1038/s41598-020-67869-8. PMID: 32612098; PMCID: PMC7330025.
- Chaabane L, Chahdoura H, Mehdaoui R, Snoussi M, Beyou E, Lahcini M, V Baouab MH. Functionalization of developed bacterial cellulose with magnetite nanoparticles for nanobiotechnology and nanomedicine applications. Carbohydr Polym. 2020 Nov 1;247:116707. doi: 10.1016/j.carbpol.2020.116707. Epub 2020 Jul 2. PMID: 32829835.
- Damodaran SP. Mesoporous Magnetite Nanoclusters as Efficient Nanocarriers for Paclitaxel Delivery. Chemistry Select. 2020; 5: 9261–9268.
- Ganesan V, Louis C, Damodaran SP. Novel Nanofluids Based on Magnetite Nanoclusters and Investigation on Their Cluster Size-Dependent Thermal Conductivity. Journal of Physical Chemistry C. 2018; 122: 6918–6929.
- Ganesan V, Lahiri BB, Louis C, Philip J, Damodaran SP. Size-controlled synthesis of superparamagnetic magnetite nanoclusters for heat generation in an alternating magnetic field. J Mol Liq. 2019; 281: 315–323.
- Chi ZL, Yu GH. Nanozyme-mediated elemental biogeochemical cycling and environmental effects. Sci China Earth Sci. 2021; 64: 1015-1025.
- Gao L, Fan K, Yan X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics. 2017 Jul 22;7(13):3207-3227. doi: 10.7150/thno.19738. PMID: 28900505; PMCID: PMC5595127.
- Reiner K. Catalase Test Protocol. 2013; 1–9.
- Kong D, Wilson LD. Synthesis and characterization of cellulose-goethite composites and their adsorption properties with roxarsone. Carbohydr Polym. 2017 Aug 1;169:282-294. doi: 10.1016/j.carbpol.2017.04.019. Epub 2017 Apr 13. PMID: 28504147.
- Wroblewski C, Volford T, Martos B, Samoluk J, Martos P. High yield synthesis and application of magnetite nanoparticles (Fe3 o4). Magnetochemistry. 2020; 6: 1–14.
- Zanata L, Tofanello A, Martinho HS, Souza JA, Rosa DS. Iron oxide nanoparticles–cellulose: a comprehensive insight on nanoclusters formation. J Mater Sci. 2022; 57.
- Fan K, Wang H, Xi J, Liu Q, Meng X, Duan D, Gao L, Yan X. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem Commun (Camb). 2016 Dec 22;53(2):424-427. doi: 10.1039/c6cc08542c. PMID: 27959363.
- Seabra AB, Paula AJ, Durán N. Redox-enzymes, cells and micro-organisms acting on carbon nanostructures transformation: a mini-review. Biotechnol Prog. 2013 Jan-Feb;29(1):1-10. doi: 10.1002/btpr.1673. Epub 2013 Jan 17. PMID: 23225699.
- Zhang R, Yan X, Fan K. Nanozymes Inspired by Natural Enzymes. Acc Mater Res. 2021; 2: 534–547
- Francisquini E, Schoenmaker J, Souza JA. Nanopartículas Magnéticas e suas Aplicações. Química Supramolecular e Nanotecnologia. 2015; 269–289.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
