Annals of Marine Science
Singlet-excited dioxygen O2(a1Δg) and organic pollutants in marine waters beneath the Sun
BF Minaev1,2*
2Department of Physics and Astronomy, Uppsala University, Uppsala, Sweden
Cite this as
Minaev BF (2023) Singlet-excited dioxygen O2(a1Δg) and organic pollutants in marine waters beneath the Sun. Ann Mar Sci 7(1): 025-033. DOI: 10.17352/ams.000035Copyright License
©2023 Minaev BF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The ground state dioxygen has a triplet spin state O2(X3Σg). The singlet excited O2(a1Δg) dioxygen possesses an excess energy of 22 kcal/mole and is highly reactive with respect to organic matter since all organic molecules have also singlet ground states with all spins paired; their reactions with O2(a1Δg) are not forbidden by spin selection. The chromophoric pollutants in sea waters under sun irradiation can generate O2(a1Δg) and other reactive oxygen species which could oxidase many wastes. This review describes mechanisms of O2(a1Δg) interaction with organic pollutants in seawater with black carbon dispersion and with corals.
Introduction
Dioxygen is everywhere – in the air, water, green plants, fishes, and inside us. This small diatomic O2 molecule saves all alive creatures from the dangerous solar ultraviolet radiation (by the UV absorption at 175 - 200 nm in the Schumann-Rung O2 band [1,2]), creates an ozone stratospheric layer that protects us from the soft UV light (240 nm), and provides respiration energy for all aerobic cells [2]. The other important role of the O2 molecule is its ability to generate the Reactive Oxygen Species (ROS), the singlet dioxygen, superoxide ion, hydroxide and peroxides, which control aerobic metabolism and cell proliferation [3-5]. Organic stuff as components of alive matter (proteins, lipids), black carbon, polyphenols, sugars, etc. consists of polymers and molecules, which possess an even number of electrons. All of them being paired with anti-parallel spins provide zero total magnetic moments; that is why most organic species represent diamagnetic substances (they are slightly repelled by an external magnetic field while the paramagnetic O2 is attracted according to Faraday’s discovery) [2,3]. Both paramagnetic and diamagnetic substances do not support their magnetization when the magnetic field is switched off. Both types of species, paramagnetic O2, and organic molecules, are chemically stable at ambient conditions (in the absence of fire) but start furiously reacting in the presence of radicals induced by spark or match [2-5].
Because of its relatively high solubility molecular oxygen (dioxygen, O2) is an important component of any aquatic environment including all marine waters. In the sun-light elucidated aquatic media excited dioxygen could be an indicator of water pollution by organic wastes which include chromophore groups. Such organic dyes usually show high biological activity being toxic and supporting bacterial environment; at the same time, they are photo-active sensitizers of the singlet dioxygen O2(a1Δg). Sea waters beneath the sun generate O2 by photosynthesis from algae and bacteria to a great extent; this complicated process is spin-forbidden. Weak spin-depending perturbations help the chloroplasts to overcome the spin restriction. A Dioxygen molecule contains 16 electrons; two of them possess non-paired spins. Thus, the ground state dioxygen has a triplet spin state O2(X3Σg). The singlet excited state O2(a1Δg) dioxygen (with all spins paired) possesses an excess energy of 22 kcal/mole and is highly reactive with respect to organic matter since all organic molecules have singlet ground states; their reactions with singlet O2(a1Δg) are not forbidden by spin selection. The chromophoric organic pollutants in sea waters under sun irradiation can generate O2(a1Δg) reactive species which could oxidase many organic wastes.
Before the study of organic pollutants in seawater, let us consider some fundamental aspects of quantum physics and remind ourselves that an electron is a tiny magnet whose magnetic moment (determined by a spin quantum number S=½) has a pure quantum origin without analogy in the visible macroscopic world. The electron spin arrow in contrast to the classical magnet has only two possible orientations (Ms=±½). For any molecule, the electronic wave functions are always eigen-functions [2] of the total spin operator .
For an even number of electrons, the S quantum number may be equal to zero (singlet ground state for the majority of stable molecules); the lowest excited state usually is a metastable state in this case with S = 1 (triplet state). The unusual paramagnetic character of dioxygen was explained in terms of quantum mechanics in 1928 by R.S. Mulliken on the basis of molecular orbital (MO) arguments [6]. For the two non-paired electron spins occupying the degenerate πg-MO it was shown by Mulliken that the triplet X3Σg- state of the O2 molecule is the lowest ground term while two singlet states, O2(a1Δg) and O2(b1Σg+), are the low-lying excited terms [6]. They have been detected soon in the night sky emission with an energy of 22 and 36 kcal/mole above the ground term, respectively [2]. The O2(a1Δg) is the first excited term of dioxygen being doubly degenerate. Because of strong spin prohibition for its return to the triplet ground state a1Δg → X3Σg- it is a metastable state. The singlet dioxygen O2(a1Δg) has a relatively long lifetime and is rather reactive with respect to diamagnetic organic molecules since both reactants are in the singlet states and their chemical interaction is spin-allowed to produce singlet state products (usually complete oxidation of all organic matter leads to diamagnetic products H2O, N2, and CO2 with zero spins) [3]. We have to mention here that the singlet O2(a1Δg) dioxygen is not powerful enough to oxidase all organics, however; it is too substrate-dependent in order to mineralize all dissolved organic material (Scheme 1).
But from a very general perspective, this simple O2 molecule essentially differs from the great majority of other chemical species by its magnetic, chemical and optical properties because of the inverted position of the triplet and singlet states. Almost all stable organic molecules have the singlet ground state and triplet – the first excited state; these two circumstances provide a great puzzle for the whole aerobic life phenomena on the Earth planet [3]. Photosynthesis creates triplet dioxygen from carbon dioxide and water by the chlorophyll catalysis in green plants beneath the Sun [1]. Aerobic cell oxidases glucose by triplet dioxygen back to H2O, and CO2 molecules with zero spins provide energy for life deposited in the singlet state Adenosine Triphosphate (ATP) diamagnetic storage, Eqs. (1):
Both processes are in the background of aerobic life, Eqs. (2), are spin prohibited [2,7]. We used to take free O2 and our respiration for granted and do not care much about the spin of dioxygen [3]; although, we have to care to understand the deep backgrounds of life, diseases, and brain machinery. Unfortunately, not many scientists care too much, and modern biochemistry skips the analysis of such great problems [5]. On the other hand, all organic Molecules (M) as diamagnetic species cannot react with the triplet O2(X3Σg) dioxygen since such reaction processes are completely spin-forbidden; from general chemistry, we know that the final products (P) of such possible complete oxidation (P = H2O, N2, CO2) are also singlet-state diamagnetic molecules like M [1,5]:
M (↑↓) + O2(X3Σg) (↑↑) ≠ P (↑↓) (↑↓) (3)
The Triplet-Singlet (T-S) transition has to occur in the reaction of Eq. (3); that is one spin-flip is necessary to complete obviously such a reaction. Such flip of the spin magnetic moment of one electron (T-S transition) in Eq. (3) could be induced solely by magnetic interaction: by the external field or by Intrinsic Magnetic Perturbations (IMP) [3]. It is well-known that IMP is very weak; they are commonly negligible in organic chemistry [2] and the external field is not necessary for oxidation by air. The typical way how reaction (3) usually proceeds in the 21% - dioxygen atmosphere – is combustion in the open air. It proceeds through the branched radical-chain reaction mechanism, Eq. (4), and is initiated by Radicals (R), the reactive molecular fragments with one non-paired electron created by the flame of a match [5]
M (↑↓) + O2(X3Σg)(↑↑) + R (↓) = Pꞌ (↑↓) (↑↓) + Rꞌ (↑) (4)
The newborn radical Rꞌ(↓) could react again with O2 (↑↑) without any spin-prohibition in a new collision (where the radical Rꞌ(↑) spin memory is lost) and the intermediate diamagnetic Pꞌ product could continue the chain reaction with the Rꞌ radical in such a way that the whole chain leads to final products P (H2O, N2, CO2) of oxidation of all organic fuels. But who brings the match in reactions of Eqs. (2) to overcome spin-prohibition in alive cells? Of course, they proceed by another mechanism that differs from the radical-chain reaction path; otherwise, the radicals would burn the aerobic cell [2]. Organic free radicals can be hazardous for living cell species at such high concentrations which are even lower than those typical for combustion flame; they can damage not only lipids but the whole mitochondria [3-5]. Meanwhile, at lower concentrations, the singlet dioxygen O2(a1Δg) and superoxide anion-radical (O2−•), like other ROS can play an important role as regulatory mediators in the cell signaling processes [4]. The ROS-mediated response is able to reestablish the whole “redox homeostasis” and also protect aerobic cells against oxidative stress [1-5]. The role of singlet dioxygen O2(a1Δg) in the ROS-mediated reactions is so important for aerobic life that it was really strange until nowadays to neglect the O2(a1Δg) reactions in the environment pollution as well as in the ecology problems of the whole biosphere [3,8].
The advent of singlet dioxygen to the photochemistry of organic solvents
In 1902 Oscar Raab discovered that the dye-colored cells had perished upon lighting and called this phenomenon “photodynamic action” (PDA) (in order to distinguish PDA from photo-sensitization in photography) [9]. Soon it was shown that the presence of dioxygen is necessary for PDA observation and the light wavelength for the PDA excitation coincides with the dye absorption spectrum [9].
The primary stage of PDA was long proposed to be similar to the well-known photooxidation observed in organic solvents [9]. The first hypotheses of primary “moloxide”, and “active oxygen” became popular before the time of the second world war (SWW); G. Schenck divided such photoreactions into two types: type I includes those reactions which the primary stage presents photo-dehydrogenation of the substrate of oxidation; type II includes reactions of dioxygen transfer with the primary stage of complex (“moloxide”) formation between O2 and the excited dye [9].
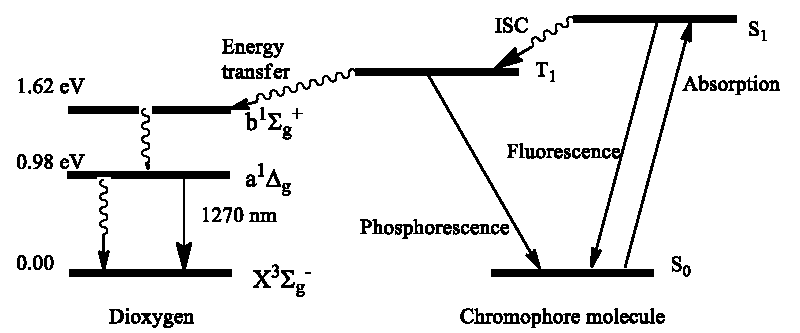
The ability of dioxygen to quench the phosphorescence (T1 →S0) [10] and fluorescence (S1 →S0) [11] of numerous dyes by the energy-transfer process through an “activated” singlet state of dioxygen was proposed to explain by H. Kautsky [11] and A. Terenin [10] during the SWW time. The formation of an “activated” excited state of dioxygen O2 (a1Δg) (↑↓) in numerous photosensitized reactions was recollected and confirmed thirty years later by C. Foot and S. Wexler [12]. Instead of “moloxide” and other hypothetic “activated” dioxygen the photosensitized reactant O2 (a1Δg) was firmly established [12].
Thus, we know that the singlet O2(a1Δg) dioxygen is naturally produced by the photochemical sensibilization mechanism through elucidation of organic dyes, pigments (or other ) with subsequent energy transfer to the triplet ground state O2(X3Σg) molecule [12]. The organic dye first absorbs light by the S0→Sn transition which colors the solvent and then typically undergoes intersystem crossing (ISC), the S1→T1 non-radiative transition to the lowest excited T1 state [8-12]. The late is metastable state since the following relaxation, radiative and non-radiative T1→S0 transition to the dye ground state, is forbidden by spin selection and by the large ΔE(T1-S0) energy gap [2]. In liquid media, the T1 lifetime is more than μs which is enough to undergo the dye-O2 collisions during which the energy transfer process occurs [10]
T1 (↓↓) + O2 (X3Σg) (↑↑) = S0 (↑↓) + O2 (a1Δg) (↑↓) (5)
Eq. (5) was first proposed by A. Terenin as additional support to the triplet origin of phosphorescence [10]. The total spin quantum number (S) in the left and right parts of Eq. (5) is the same (S = 0) and the reaction is spin-allowed; thus, the energy transfer from the T1 state of excited dye to dioxygen occurs with the singlet O2(a1Δg) generation [10,13]. Thus, the most efficient PDA effect includes light absorption by a dye molecule (S1←S0) with subsequent ISC (S1→T1) providing a long-lived triplet excited dye that collides with abundant O2 (X3Σg) dioxygen in an aqueous solvent with dye-colored cells; such collisions lead to energy transfer expressed by eq. (5) and generation of the singlet O2(a1Δg) state. The late has no spin restriction for oxidative reaction with the singlet ground states of organic components of the cell and such sensitized photo-oxidation destroys the cell organelles leading to cell death in living creatures which was first observed by Oscar Raab. As a spin-active reactant O2(a1Δg) is a good oxidizer of many unsaturated organic molecules [12,13]. Because of its long lifetime (3 μs in water), high selectivity with respect to electron-rich DOM, and wide pH tolerance, the singlet O2(a1Δg) dioxygen attracts great attention in water purifying from various organic pollutants [13-23].
Singlet dioxygen as a photochemically sensitized reactive intermediate and ubiquitous oxidant in sunlit aquatic environments
Many dyes are present in the sea waters; besides exogenous pollutants, there are Green Fluorescent Proteins (GFP), chlorophylls and numerous pigments of chemiluminescent plankton. A few remarkable examples of auto-fluorescent plants present such senescent leaves like Spathiphyllum wallisii which accumulates chlorophyll catabolites that emit blue fluorescence, the Ginkgo biloba accumulates hydroxy kynurenic acid in a high concentration (this alkaloid being derived from the catabolism of tryptophan provides very strong blue fluorescence) [14]. The triplet dioxygen O2 (X3Σg) can quench such fluorescence, Eq. (5), and transform to excited singlet O2(a1Δg) state of dioxygen which is a good photo-oxidant of organic matter [9-16].
Thus, the singlet O2(a1Δg) dioxygen is a photochemical reactive oxygen species produced in sunlit waters by the energy transfer from the triplet excited T1 states of natural aqueous sensitizers – fluorophores or phosphorus. It can be produced also in dark water as a result of the metabolism of numerous aqueous creatures [24]. There has been an increasing interest during recent years in the measurements of apparent O2(a1Δg) quantum yields (ΦΔ) of aquatic (and atmospheric) organic pollutants which can absorb sunlight [15]. This photochemical parameter is important for environmental pollution estimation and modeling of organic contaminants distribution in the world’s oceans. In general, studies of the ΦΔ quantum yields are necessary to advance the fundamental understanding of photophysics and photochemistry of dissolved organic matter [13-19]. The ΦΔ value represents the number of moles of O2(a1Δg) produced per moles of photons absorbed by a sensitizer [13]. The authors of Ref. [13,15] have critically evaluated the experimental and theoretical methods used to determine and explain ΦΔ parameters of organic solvents. The bulk water chemistry, molecular and spectroscopic parameters of organic chromophores, wavelength, chemical treatments, season location of natural organic matter and water pollutants were discussed in a special recent review on O2(a1Δg) yield in environmental waters [15].
The often employed mechanism for the O2(a1Δg) detection with the usage of organic fluorophores is based on the photochemically sensitized formation of endoperoxide products (chemical trap method) [12-23,25]. If an Anthracene Molecule (AM) is linked to such a fluorophore like a fluorescein derivative we can expect AM oxidation in the sunlit solvent containing natural O2 concentration.
The photochemical mechanism of this oxidation is the following (Scheme 2). The organic fluorophore is excited by sunlight as a result of the S1←S0 absorption and before it gets the possibility to emit fluorescence (S1→S0), the energy transfers to the linked AM or to the dissolved triplet O2(X3Σg) dioxygen occurs with the stepwise singlet O2(a1Δg) generation. Then, the excited singlet oxygen molecule reacts with AM, producing the characteristic 1,4-cycloaddition in the central AM ring [15]. Such cycloaddition strongly disrupts the planar structure of the former AM and hinders the photochemical kinetics of the whole fluorophore. With the greatly reduced photochemistry, the late fluorophore is able to emit light after excitation; thus, it provides a fluorescence signal in the presence of O2(a1Δg) [14]. A few examples of such O2(a1Δg) sensors used in sea plants, including the Singlet Oxygen Sensor Green, and the dansyl-based probes are discussed in ref [14,15].
The most efficient way of the O2(a1Δg) generation is realized in those dyes which show fast Intersystem Crossing (ISC), that is non-radiative S1→T1 transition (Scheme 2). In liquid media the T1 lifetime is long enough to undergo the dye-O2 collisions; the tight dye-O2 contact is necessary for proper orbital overlapping making the efficient energy transfer by exchange mechanism, Eq. (5). At the first step, energy transfer proceeds from the T1 triplet dye to the second singlet state of dioxygen O2(b1Σ+g); the late relax very fast to the lowest O2(a1Δg) state [25,26].
The singlet O2(a1Δg) generation by aquatic sunlit fluorophores leads to the chemical transformation of organic pollutants, to deactivation of pathogens, and aerosols, the sunlight-induced oxidation of natural organic matter, and to the formation and the photochemical aging of corals. To quantify the role of O2(a1Δg) in these processes, we have to assess the ability of natural fluorophores and chromophores to sensitize singlet dioxygen formation. The apparent singlet oxygen quantum yield ΦΔ is a useful photochemical parameter for this purpose [13,14]. It accounts for variations in the absorption spectra and concentration of the chromophore-sensitizer, as well as variations in the light intensity. These features make quantum yield ΦΔ a useful universal parameter in environmental chemistry since it can predict variations in the O2(a1Δg) generation and its steady-state concentrations in water as a function of sunlight intensity; that is a function of water depth, the presence of other light absorbers in water constituents, Dissolved Organic Matter (DOM) concentrations, fluctuations of seasonal light intensity, etc. [14]. ΦΔ is necessary as input parameters in the predictive models of micro-pollutants’ lifetimes and their steady-state concentrations [19-21]. Besides that, the ΦΔ quantum yields can be used also to study the sensing properties of natural DOM- chromophores. The ΦΔ value can be measured for all DOM-sensitizers depending on their origin, that is to compare the microbially or terrestrially derived origin of the dissolved organic molecules [22] and to assess the effect of environmental features (photo-oxidation) on the photo-reactivity of DOM [22,23]. The photoactivity of DOM is critical for the whole photochemistry of natural waters. At last, the ΦΔ values measurements offer the prospect of deeply understanding the fundamental photo-physical and photo-chemical properties of dissolved organic matter [13,14]. Therefore, ΦΔ is really a useful parameter in these respects.
In order to estimate ΦΔ one has to measure a number of spectra-chemical factors [8-13]. The first crucial parameter is the number of photons absorbed by the chromophore-sensitizer. According to the Buger-Beer-Lambert law, the light absorption measurement provides the molecular absorbance of the experimental solution, solvent concentration, and the optical way length. Direct measurements [25] of the dioxygen absorption coefficients (ε) in aerated water and solutions have been performed by the laser photochemistry methods; they showed that laser excitation of O2 dissolved under normal conditions has caused the oxygenation of the singlet dioxygen chemical traps such as rubrene, uric acid, and tetracene [25].
O2(X3Σg) + hν → O2(a1Δg) + Chemical trap → Trap oxygenation. (6)
The kinetic studies of the trap oxygenation rates permit to obtain the ε coefficients of dissolved dioxygen in the absorption bands at 1272 and 765 nm which correspond to O2(a1Δg) and O2(b1∑+g) excitations, respectively [25]. The dioxygen photo-activation can be observed also by detecting infrared (IR) phosphorescence of the singlet O2(a1Δg) dioxygen at 1270 nm which occurs under irradiation of the aerated solutions by the dark red light at 765 nm [25].
O2(X3Σg) + hν(765 nm) → O2(a1Δg) → O2(X3Σg) + hν(1272 nm) (7)
The dioxygen absorption coefficient at the laser wavelength 765 nm extracted from the phosphorescence measurement coincides with the ε coefficient measured by the chemical trap method [25]. The O2(a1Δg) lifetime in the solvents containing C-H or O-H groups is known to decrease by thousands of times (in comparison with CCl4 and C6F6 solvents) since vibrations of the light groups accept the singlet dioxygen excitation energy [13]. The rate of energy transfer to O-H vibrations is much faster than the IR phosphorescence of the singlet O2(a1Δg) dioxygen at 1270 nm which is completely quenched in water [26]. Thus, the quantum yield ΦΔ in aqueous media is usually measured by the chemical trap methods (not by IR phosphorescence study) [12-23,25-28].
The general role of reactive oxygen and nitrogen species in water pollution
The O2(a1Δg) as a photochemically produced reactive intermediate is an important oxidant of organic pollutants ubiquitous in sunlit aquatic environments being at the same time a transductor of chemical signals in the intercellular transduction pathways between sea creatures. Even small fluctuations of dioxygen amount can strongly influence the cells signaling functions through O2(a1Δg) and other ROSs activity [5,24] and affect the whole cellular metabolism, their proliferation, and differentiation in sea waters. Most of these ROS are paramagnetic species since they possess internal magnetic moments [24]. The O2(a1Δg) and other ROS properties crucially depend on their paramagnetism and molecular electronic structure of such chemically active and non-saturated species. Modern quantum chemistry provides useful knowledge on the spin-dependent interactions between O2(a1Δg), other ROS and organic water components [13-16,26]. This review describes the most essential spin-dependent features of the dioxygen excited states and ROS involvement in some sea-water ecology problems. Though O2(a1Δg) possesses zero spin (singlet spin state) it is still a paramagnetic species because its magnetic moment is determined by the orbital angular momentum Lz = 2ℏ [18]. Many other ROS (O2-•, OH, HO2, RO2,) as well as NO, NO2 have an odd number of electrons and S = 1/2 being radicals with the doublet ground state. The singlet state molecules, O3,H2O2, and peroxynitrite (ONO2-), also belong to ROS because of their chemical reactivity, though they have a much longer lifetime [4,14]. The ozone and peroxynitrite anion possess the low-lying triplet excited state which is important for their generation and chemical activity [18,27,29].
Accounting for the facts that the triplet excited states (T1) of the dissolved chromophoric organic molecules (DCOM) are the direct precursors of the singlet O2(a1Δg) dioxygen, eq. (5), good correlations between the formation yields of these two species are expected. Indeed, the apparent singlet O2(a1Δg) dioxygen quantum yields ΦΔ strongly correlates with the triplet quantum yield coefficient ΦT [15]. The triplet quantum yield ΦT coefficient is usually determined as the ratio of the measured rate constant of Trimethyl Phenol (TMP) reaction with DCOM and the rate of light absorption; this ratio is directly proportional to ΦT triplet quantum yield. This correlation is consistent not only with the fact that the triplet-excited DCOM* is the precursor of O2(a1Δg), but also implies that a very similar dissolved organic pollutants pool is responsible for both the oxidation of TMP and the formation of singlet O2(a1Δg) dioxygen.
Some positive correlation was also observed between ΦΔ and the measured quantum yield of organic fluorescence (ΦF) for a number of dissolved organic pollutants treated with the ozone [14,15]. This relationship was valid for the increasing ozone levels only for essentially low quantum yields; the result was interpreted in such a way that there exist two distinct pools of pollutants responsible for fluorescence and singlet O2(a1Δg) generation [15].
The ubiquitous DOM in aquatic systems is represented by dissolved Black Carbon (BC) and humic acids (HA) [19,22]. BC is a residue formed by the incomplete combustion of fossil fuels and biomass; its water-soluble fraction, known as the dissolved BC (DBC), is an important part of the whole DOM pool [22]. It was shown that ΦΔ values measured with DBC fractions (derived from various biomass stocks) are in the region 3.46% - 6.13% being much higher than those of HA substances (1.26% - 3.57%) [22]. These results support some other findings [21] that DBC is one of the most photoactive components of the whole DOM pool with respect to O2(a1Δg) generation.
Dioxygen in the ground state O2(X3Σ-g) is a chemically stable biradical with the internal magnetic moment (paramagnetic species) because of two non-paired electrons in the O2 valence shell possessing parallel spins [2,24]. The most biochemically important ROS are the excited singlet O2(a1Δg) dioxygen, superoxide ion-radical O2-•(X2Πg), and hydroxyl radical OH•(X2Π); they are short-lived paramagnetic species (for all of them the ground state term is given in parentheses [18]). The late two species are paramagnetic because of the odd number of electrons and one non-paired electron spin occupying degenerate π-shell, the singlet excited O2(a1Δg) dioxygen – because of the orbital magnetism [24].
The first excited state of dioxygen, O2(a1Δg), possesses also antiparallel spins as all organic pollutants [2]. We need to stress that this state is doubly degenerate: there are two states with different wave functions but with the same energy (22 kcal/mole above the O2(X3Σg) ground state) [1]. The degenerate character of the singlet excited O2(a1Δg) dioxygen is often neglected in many photochemical studies including environmental problems [12-15] and photodynamic therapy (PDT) [26,28]. This neglect essentially hinders the proper understanding of the sunlit water pollution problems and the O2(a1Δg) role, the origin of the fundamental electronic mechanisms behind the O2(a1Δg) sensibilization and quenching [2,28]. Degeneracy of the singlet dioxygen O2(a1Δg) state determines the orbital angular momentum Lz, paramagnetism, and magnetic moment providing a particular influence on dioxygen reactivity [17]. In the numerous ROS studies [1,4,14,15,20-23] an important paramagnetic property of the superoxide O2-•(X2Πg), and hydroxyl OH•(X2Π) radicals is underestimated; this is the unusually high Spin-Orbit Coupling (SOC) which split their degenerate 2Π doublet states [3,5,8,24]. Its chemical sequence for environmental and life science will be considered in the next chapter.
We want to note that the excited O2(a1Δg) dioxygen is not only important as an oxidant of DOM in the sunlit ocean but also as a signaling and active species in numerous biochemical reaction chains in the world ocean metabolism. The electron spin importance for the whole of biology was first discovered by L. Poling during his hemoglobin studies [1,2]. The Fe(II) ion in heme has spin quantum number S = 2; thus, heme is a paramagnetic species and its coupling with O2 is a complicated reaction that depends on the dioxygen spin state, on spin-orbit coupling and the exchange interaction between Fe(II) in hemoglobin and the molecules of the air [5,7]. Modern ecology has to describe the most essential spin-dependent features of the dioxygen O2(X3Σg) and O2(a1Δg) states, as well as those of superoxide O2-• and all ROS with their involvements in photo-interactions with organic pollutants and biochemical chains including photosynthesis and respiration of aqueous plants, fishes, corals, plankton, and other alive creatures with their mutual signaling and energy exchange.
Most biochemically induced intracellular ROS are derived from the superoxide radical O2−• which is generated by single-electron reduction of O2(X3Σg) and O2(a1Δg) dioxygen. The superoxide ion radical is transferred to H2O2 peroxide by superoxide dismutase (SOD) in the cell [24]. Singlet dioxygen O2(a1Δg) generation in dark water is typically produced through the superoxide O2−• ion oxidation [9]. In the final stage of the cycle, the Fenton reaction with aqueous Fe(II) ions leads to the hydroxyl radical OH•, the most dangerous ROS reagent [30]. Thus, the superoxide ion and its internal magnetic properties play a fundamental role in ROS production, in their oxidative reactivity, and in their numerous signaling functions. Unfortunately, the important spin-orbit coupling mechanism [2,31] which promotes superoxide O2−• anion to overcome spin prohibition of dioxygen reactivity with respect to organic matter is not accounted for so far either in the biochemistry of enzymes [32] nor in the environment sciences [15,22].
Spin-orbit coupling importance for reactive oxygen species
Aquatic organisms use dissolved O2 for respiration to produce ATP molecules through the Crebs cycle of oxidative phosphorylation in mitochondria [1]. Defects of electron-transport chains in mitochondria lead to possible predictable dysfunctions in most tissues and in signaling systems [5]. One should note that the presence of paramagnetic metal ions of Fe(II) and Mg (II) in cytochrome c oxidase and in chlorophyll, respectively, explains how reactions of Eq. (2) can overcome the spin prohibition [2,5,24]. Paramagnetic metal ions provide their non-paired spins in these enzymes in order to activate triplet dioxygen for oxidation, Eq. (2); in a way quite similar to a combustion reaction, Eq. (3). The driving force for such activation is intermolecular exchange interaction between various non-paired spins in different parts of the enzyme [2]. (The great difference from Eq. (3) is that radicals in combustion lost their spin memory in different collisions; but, RP in enzymes are fixed together [5]). Failure in the electron-transport chain can lead to the failure of cytochrome c oxidase enzyme and in the final O2 transformation to water. Such defects are accompanied by ROS production including O2(a1Δg) [3].
Numerous enzymes without metals are able to activate dioxygen in the cell [33]. For example, oxidative flavin- and pterin-dependent enzymes are ubiquitous in alive matter [32]. Their π-aromatic systems can undergo fast electron transfer so demonstrating a reach redox activity [3,24,30-35]. Various redox stages of flavins and pterin provide the so-called “proton-coupled electron transfer” processes which are important in numerous biochemical functions, such as oxidation, biosynthesis, detoxification, and biodegradation [24,30,32]. During bio-reactions catalyzed by flavin-dependent enzymes various forms of transient radical pair (RP) can be generated (including, for example, FADH•…O2-• radical pair [31]). In this case, the reduced flavin (Flred) is typically oxidized to semiquinone (Flsq•) radical upon interaction with O2 followed by flavoperoxide FlOOH subsequent formation [31]. V. Massey postulated a single-electron transfer from the reduced flavin (Flred) to dioxygen O2(X3Σg) with subsequent intermediate triplet RP formation between superoxide O2-• ion and semiquinone radical Flsq• in such a form [34]:
Flred(↓↑) +O 2(↑)(↑) → [Flsq• ↑↑ O2-•] [Flsq• ↑↓ O2-•]→FlOO- FlOOH (8)
In such enzyme active center the spin-inversion occurs at the triplet ↑↑ RP phase, which has to undergo the Triplet-Singlet (T-S) transition in order to produce the diamagnetic FlOOH product, Eq. (8). As an example, glucose oxidase (GO) can be considered [31]. V. Massey not commented on the magnetic origin of the forces which are responsible for the T-S spin-transition in Eq. (8) [24,31]. However, we can suspect that V. Massey had kept in mind the ideas of Radical Pair Theory (RPT) [36] which was very popular twenty years ago being applied for Magnetic Field Effects (MFE) observed in radical chemical reactions [5]. The RPT considers the probability of T-S transition [24,36] which is induced by Hyperfine Interaction (HFI) between electron and nuclear spins in radical reaction. This mechanism of T-S transition is possible only in the spatially separated radicals inside a non-bound RP in the solvent cage [18,36]. The T and S states of such non-bound RP possess the same energy (they are degenerate); that is why even a very weak HFI according to RPT can produce the T-S spin transition [24]. The RP theory could be applied to O2 interaction with a free flavin in the aqua solvent; however, it could not apply to a real oxidative enzyme where FADH and O2-• are kept tightly bound in the active center (like in GO) [3,24]. The origin of the driving force for the T-S spin transition in Eq. (8) is a fundamental problem of O2 activation in living matter by numerous enzymes which are free of metal cofactor [33]. Unraveling this mechanism of O2 activation could be very important not only for enzymology but also for many practical biomedical and ecology applications[13-23,25-28] , including also O2(a1Δg) generation in dark water and DOM oxidation.
The extremely weak nuclear hyperfine perturbations cannot induce a fast and competitive triplet-singlet spin flip in radical pair of the type shown by Eq. (8) [37,38]. The T-S transition rate constant has to compete with the rate of the triplet RP dissociation in Eq. (8). Such RP dissociation will lead to the escape of the active superoxide-ion from the enzyme active center and its release into the water environment or cytoplasm. That is why the understanding of the driving force origin for the T-S transition in Eq. (8) is such important for the oxidation of DOM pollutants by various forms of active oxygen (all being released through superoxide ion; both O2(a1Δg) and O2(X3Σg) can be released by electron detachment from superoxide O2-• ion).
An explanation of the T-S transition in Eq. (8) has been proposed 20 years ago [31] but it is still not known by a wide scientific community of biochemists and ecologists [32]. It is based on account of two possible electronic configurations in the degenerate open shell (πg)3 of the O2-• superoxide ion: (↑)(↓↑) and (↓↑)(↓) in Eq. (9); therefore, the T-S transition in the radical pair, Eq. (8), can be presented as
3[Flsq•(↑)… O2-• (↑)(↓↑)] 1[Flsq•(↑)… O2-• (↓↑)(↓)] (9)
Here the brackets near the O2-• ion denote two degenerate molecular orbitals πg,x, and πg,y of dioxygen [31]. The arrow in Eq. (9) denotes electronic Spin-Orbit Coupling (SOC) as a driving force of the spin torque [5]. The left upper symbols in Eq. (9) denote the triplet and singlet states, as usual; these states differ by the electronic configurations inside the superoxide O2-• ion whereas the spin of flavin semiquinone radical is the same in the left and right parts of Eq. (9); thus, flavin cofactor does not participate in magnetic activation and does not enhance the SOC matrix element. The T-S transition in Eq. (9) includes the orbital rotation πg,x → πg,y with its simultaneous spin flip in superoxide O2-•. This orbital rotation creates a magnetic torque which is responsible for the spin T-S transition [31]. The strong SOC in Eq. (9) is equal to ½ Aso, where Aso is the ground state O2-•(X2Π) SOC constant [2,39]. According to approximation [2], the Aso(X2Π, O2-•) constant coincides with the SOC splitting (ζO) in the oxygen atom ground state O(3P) being close to a value of 160 cm-1 [2]. Such analysis coincides well with experimental data on the fine structure of the O2-• ion spectrum [39]. This SOC energy is a thousand times larger than the nuclear-electron HFI in the radical pair theory [36-38].
Superoxide O2-• ion-radical can escape from oxidative enzymes and destroy mitochondria if the T-S spin-flip in Eq. (9) is not fast enough to compete with the dissociation of the radical pair described by Eq. (8). The rate of the spin-flip (kT-S) can deviate from the normal working regime determined mostly by the square of the Aso(X2Π, O2-•) constant since kT-S depends also on the hindered rotation of the O2-• radical in the cage of enzyme active center and on the low-frequency vibration in the nearest protein scaffold environment [5,28]. The late factors could be unsteady and alter in the flexible protein scaffold; thus, the kT-S rate constant also can fail in competition with the RP dissociation rate [5]. All these enzymatic processes are relevant to O2(a1Δg) generation in seawaters in the presence of numerous algae, bacteria, and other biological sources.
The singlet excited dioxygen O2(a1Δg) and superoxide O2-• (X2Πg) radicals are the most important ROS [3,5,8]. Excessive generation of ROS and oxidative stress play a crucial role in the pathogenesis of many diseases and signaling chains work [5,24,36]. Not surprising would be their activity in such a rich environment as the ocean waters.
Advanced Oxidation Processes (AOP) are designed to remove organic materials in water (including wastewater) by DOM oxidation through their reactions with hydroxyl radical [22,40]; AOP employs such highly reactive ROS as •OH radical in order to diminish the amount of a wide range of organic pollutants in water with diffusion-controlled kinetics [40]. Since hydroxyl radical has a short lifetime (τ<μs), it is generated during ozone- and other UV-induced processes in situ by activating a stable H2O2 precursor of hydroxyl. The peroxide reacts with dissolved Fe(II) ion by the Fenton reaction and provides •OH radical in situ [22,30]. Similar processes can occur with the help of superoxide O2-• ion naturally generated by sea plants and animals. We have to remind that the singlet excited dioxygen O2(a1Δg) can be produced by the single-electron detachment (oxidation) from the superoxide O2-• (X2Πg) radical; for example by the scheme: O2-• (↓↑)(↓) – e(↓) = O2(a1Δg) (↓↑).
There are many other effective catalytic photo-techniques of water pollutants photo-degradation [17,29,38,41,42]. In the laboratory the singlet dioxygen O2(a1Δg) is often detected in organic solvents through its extremely weak near-IR luminescence (1.27 μm) in the singlet-triplet transition O2(a1Δg)→O2(X3Σg) [13] which is triply prohibited by two orbital selection rules and also by the spin-selection [2]. Quantum-chemical calculations of the T and S state wave functions of the dioxygen electronic open shell O2(πg)2 and their spectral analysis provide fundamental knowledge about the role of intrinsic magnetic interaction (SOC) which makes it possible to overcome orbital and spin prohibitions in such fundamental phenomena like oxidation and light emission by dioxygen [2,28]. The first explanation of Eq. (9) was obtained during the spectral problem study [43] connected with the singlet dioxygen O2(a1Δg) quenching. The journey from O2(a1Δg) spectra to enzymology and ecology takes a long time [3,43].
The insight into the ocean ecology from the singlet dioxygen perspective could be completed by comparison of the ROS role in their photo-chemical interaction with alive and non-alive organic matter. Aerobic respiration, Superoxide Dismutase (SOD), and photosynthesis occur on the Earth about 2.5 billion years ago [3,42]. The geological history of aerobic evolution recorded by various fingerprints on mountain rocks shows that ROS occur since the very old times of the photosynthetic era. These records indicate the “strategic” role and primordial importance of the spin prohibition for the O2(X3Σg) reactions with organic matter and with sunlight [8,24]. The triplet nature of the dioxygen O2(X3Σg) molecule and its spin restrictions explain clearly why our world had not burnt down during the Great Oxygenation Catastrophe (GOC) billions of years ago [44] when the green-blue algae started to fulfill our atmosphere by dioxygen. The primordial anaerobic bacteria had perished during GOC; they had been substituted by eukaryotes which started a more efficient type of evolution [24,44]. The role of mitochondria extends far beyond oxidative phosphorylation in the cell; it concerns their involvement in ion homeostasis and apoptosis through the signaling function of O2(a1Δg) and other ROS. They occurred on the Earth almost simultaneously with photosynthesis and dioxygen [4,5]. The high level of ferrous ions Fe(II) in the primordial ocean provided O2 reduction and superoxide O2-• generation. The O2-• late radical could dismutate to form hydrogen peroxide; then, H2O2 interacted with soluble Fe(II) ions by the Fenton reaction [30] and produced the highly reactive OH• radical [4]. All these active molecules, together with singlet O2(a1Δg) dioxygen, produced by DOM and natural dyes beneath the Sun, constitute the highly aggressive ROS. Thus, the ROS occurred naturally at the GOC beginning, and later they plaid an important role in aerobic life evolution. It was shown [4] that SOD is the oldest enzyme on the planet; it was developed by evolution for ROS scavenging to avoid oxidative stress.
Superoxide dismutase has been found in all known kingdoms of life [4]; as geological records show they evolved in the GOC time earlier than the differentiation that happened between archaea and eukaryotes [1,4]. The GOC was the first ecological disaster that strongly shacked the whole planet; thus, GOC left many geology records that clearly show the history of the SOD enzymes in the early Earth’s time. The role of spin factors and paramagnetic properties in the ROS and SOD chemical activity including the SOC effects are described in several recent reviews [2,3,5,7-9,13,16-18,24,26,28,35].
The singlet dioxygen and other reactive oxygen species are involved in multiple endogenous and environmental challenges. We know nowadays that the electron transfer and SOC effects can influence the stepwise quantum transitions between different forms of dioxygen, ROS radicals, and birdcalls: O2(X3Σg) → O2(a1Δg) → O2-•(X2Πg) → OH•(X2Π1/2) → DOM oxidation. New insights into the role of spin-dependent effects and paramagnetic properties of dioxygen and ROS could help to enhance the natural photo-chemical process of DOM degradation and open new strategies for water purification. The role and mechanisms of vibronic relaxation which accompanies spin transitions in the radical pairs of enzymes, Eq. (9), and in the quenching process of singlet O2(a1Δg) dioxygen [28,41] deserve more attention in this respect.
Conclusion
Organic chromophore molecules dissolved in surface waters are involved in multiple photochemical processes which are crucial to the degradation of organic contaminants, dissolved black carbon and humic acids, and to the whole global carbon recycling processes. Under solar irradiation, these chromophores are excited into the triplet T1 state that further reacts with dissolved O2(X3Σg) molecules to generate a series of reactive paramagnetic intermediates, such as O2(a1Δg) dioxygen, hydroxyl, and superoxide radicals, etc. Environmental impacts of these ROS have received considerable attention with respect to effluent organic matter which is composed of a combination of natural organic background, soluble microbial fractions, and trace amounts of organic pollutants. Comparison with O2 activation in the live matter is very promising for ecology since it prompts common effective mechanisms for both problems. Aerobic respiration and photosynthesis being the totally spin-forbidden processes are activated by paramagnetic metal ions with exchange perturbations which are also useful in ROS generation. Other numerous oxygenation processes are catalyzed by enzymes without the assistance of metal ions. They act by electron transfer step from the organic substrate or cofactor to dioxygen with superoxide ion O2-• generation. The T-S spin-flip occurs at the Radical Pair (RP) steps being induced by spin-orbit coupling inside the superoxide anion. Similar RP steps may operate in the soluble DOM pollutants. This SOC mechanism is widely spread in redox O2 biochemistry. Magnetic torque in the superoxide ion is one of the main driving forces for ROS production and O2 activation in oxidative processes. The SOC effect and electron transfer can influence quantum transitions between different forms of dioxygen, ROS radicals, and birdcalls: O2(X3Σg) → O2(a1Δg) → O2-•(X2Πg) → OH•(X2Π1/2). New insights into the role of spin-dependent effects and paramagnetic properties of dioxygen and ROS could help to classify various water cleaning techniques.
This work was supported by the Ministry of Science and Education of Ukraine (project 0122U000760) and by the Swedish Wenner-Gren Foundations (project GFU 2022-0036). The authors express gratitude to Dr. Hans Ågren, Dr. V.A. Minaeva, and Dr. L.B. Yaschuk for useful discussions.
- Lane N. Oxygen. The Molecule that Made the World. Oxford: University Press, 2002; 365.
- Minaev BF. Electronic mechanisms of molecular oxygen activation. Russian Chemical Review. 2007 Nov; 76(11): 988–1010. PMID: 15040259. DOI: 10.1038/nrmicro821.
- Minaev BF. Magnetic torque inside the superoxide radical is the driving force for oxygen activation by dioxygenases. Advances in Chemistry Research. Nova Science Publishers, Inc. 2022; 75.
- Mittler R. ROS are good. Trends in Plant Science. 2017; 22(1): 11–8. PMID: 27666517. DOI: 10.1016/j.tplants.2016.08.002.
- Minaev B. The spin of dioxygen as the main factor in pulmonology and respiratory care. Arch Pulmonol Respir Care. 2022 Dec; 8(1): 028-033. DOI: https://dx.doi.org/10.17352/aprc.000081.
- Mulliken RS. Oxygen triplet spin state. Nature. 1928; 122: 505-507.
- Minaev BF, Minaeva VA. Spin-dependent binding of dioxygen to heme and charge transfer mechanism of spin-orbit coupling enhancement. Ukrainica Bioorganica Acta. 2008; 2(1): 56-64.
- Minaev B. Magnetc torque in superoxide ion is the main driving force of dioxygen activation in aerobic life. Biomed J Sci Tech Res. 2021; 38(4): 121-131. DOI: 10.27717.BJSTR.MS.ID.006171.
- Minaev B. Photochemistry and spectroscopy of singlet oxygen in solvents. Recent advances which support the old theory. Chemistry & Chemical Technology. 2016; 10: 519-530.
- Terenin A. Photochemical Processes in Aromatic Compounds. Acta Physicochim. URSS. 1943; 28: 210−241.
- Kautsky H. Quenching of Luminescence by Oxygen. Trans Faraday Soc. 1939; 35: 216–219. DOI: 10.1039/tf9393500216.
- Foote CS, Wexler S. Singlet Oxygen. A Probable Intermediate in Photosensitized Autoxidations. J Am Chem Soc. 1964; 86: 3880-3881. DOI: 10.1021/ja01072a061.
- Bregnhøj M, Westberg M, Minaev BF, Ogilby PR. Singlet Oxygen Photophysics in Liquid Solvents: Converging on a Unified Picture. Acc Chem Res. 2017 Aug 15;50(8):1920-1927. doi: 10.1021/acs.accounts.7b00169. Epub 2017 Jul 21. PMID: 28731691.
- Ortega-Villasante C, Burén S, Blázquez-Castro A, Barón-Sola Á, Hernández LE. Fluorescent in vivo imaging of reactive oxygen species and redox potential in plants. Free Radic Biol Med. 2018 Jul;122:202-220. doi: 10.1016/j.freeradbiomed.2018.04.005. Epub 2018 Apr 5. PMID: 29627452.
- Ossola R, Jönsson OM, Moor K, McNeill K. Singlet Oxygen Quantum Yields in Environmental Waters. Chem Rev. 2021 Apr 14;121(7):4100-4146. doi: 10.1021/acs.chemrev.0c00781. Epub 2021 Mar 8. PMID: 33683861.
- Minaev B. Environment friendly spin-catalysis for dioxygen activation. Publishing House of Lviv Polytechnic National University, Chemistry & Chemical Technology. 2010; 6: 1-17.
- Norouzi A, Nezamzadeh-Ejhie A. α-Fe2O3/Cu2O heterostructure: Brief characterization and kinetic aspect of degradation of methylene blue. Physica B. 2020; 599: 412422.
- Khudyakov IV, Minaev BF. Molecular Terms of Dioxygen and Nitric Oxide. Physchem. 2021 Jul; 1(2): 121-132.
- Litvin VA, Minaev BF. Spectroscopy study of silver nanoparticles fabrication using synthetic humic substances and their antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2013 May;108:115-22. doi: 10.1016/j.saa.2013.01.049. Epub 2013 Feb 6. PMID: 23466321.
- Latch DE. The Role of Singlet Oxygen in Surface Water Photochemistry. In Surface Water Photochemistry; The Royal Chemical Society. 2015; 139–165. [Crossref], Google Scholar
- Schwarzenbach RP, Gschwend PM, Imboden DM. Indirect Photolysis: Reactions with Photooxidants in Natural Waters and in the Atmosphere. In Environmental Organic Chemistry; John Wiley & Sons, Inc., 2002; 655-686.
- Du Z, He Y, Fan J, Fu H, Zheng S, Xu Z, Qu X, Kong A, Zhu D. Predicting apparent singlet oxygen quantum yields of dissolved black carbon and humic substances using spectroscopic indices. Chemosphere. 2018 Mar;194:405-413. doi: 10.1016/j.chemosphere.2017.11.172. Epub 2017 Nov 30. PMID: 29223811.
- Bodhipaksha LC, Sharpless CM, Chin YP, MacKay AA. Role of effluent organic matter in the photochemical degradation of compounds of wastewater origin. Water Res. 2017 Mar 1;110:170-179. doi: 10.1016/j.watres.2016.12.016. Epub 2016 Dec 13. PMID: 28006707.
- Minaev BF. Dioxygen and reactive oxygen species’ paramagnetic properties are important factors in dermatology. Int J Dermatol Clin Res. 2022; 8(1): 016-023. DOI: 10.17352/2455-8605.000046
- Krasnovsky AA, Kozlov AS, Benditkis AS. Laser activation of oxygen in aerated solvents. Measurement of the absorption spectra of oxygen dissolved in aerated solvents under natural conditions. Russian Physics Journal. 2022 Mar; 64 (11) 2035-2045. DOI 10.1007/s11182-022-02552-1.
- Minaev BF, Murugan NA, Ågren H. Dioxygen spectra and bioactivation. International Journal of Quantum Chemistry. 2013 Jul; 113 (14): 1847-1867. DOI: 10.1002/ qua.24390.
- Minaev BF, Ågren H. The interpretation of the Wulf absorption band of ozone. Chem Phys Let. 1994; 217(5-6): 531-538. https://doi.org/10.1016/0009-2614(93)E1445-M
- Minaev BF. Spin-orbit coupling mechanism of singlet oxygen a1Δg quenching by solvent vibrations. 2017 Feb; Chemical Physics 483(1): 84-95. doi.org/10.1016/j.chemphys.2016.11.012
- Minaev BF, da Silva M, Panchenko O, Ågren H. Prediction of a new spin-forbidden C’’5Πu → B3Πg transition in the N2 molecule. J Quant Spectr Rad Transfer. 2021 (in press).
- Zakharov II, Kudjukov KY, Bondar VV, Tyupalo NF, Minaev BF. DFT-based thermodynamics of Fenton reactions rejects the ‘pure’ aqua complex models. Comput Theor Chemistry. 2011 Jan; 964(1-3): 94-99.
- Minaev BF. Spin effects in reductive activation of O2 by oxidase enzymes. RIKEN Review. Tokyo. 2002 Feb; 44: 147–9.
- Romero E, Gómez Castellanos JR, Gadda G, Fraaije MW, Mattevi A. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chem Rev. 2018 Feb 28;118(4):1742-1769. doi: 10.1021/acs.chemrev.7b00650. Epub 2018 Jan 11. PMID: 29323892.
- Minaev BF. How cofactor–free oxygenases can overcome spin prohibition in substrates oxygenation by dioxygen. Chem. Physics. 2019 May; 251(1): 61–68. DOI: 10.1016/j.chemphys.2019.01.021.
- Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994 Sep 9;269(36):22459-62. PMID: 8077188.
- Minaev BF, Minaeva VA, Agren H. Spin-orbit coupling in enzymatic reactions and the role of spin in biochemistry. In: Leszczynski J, editor. Handbook of Computational Chemistry: Springer Berlin/ Heidelberg. 2012 Sep; 1067–1093. DOI: 10.1007/978–94-007-0711–5_29.
- Zadeh-Haghighi H, Simon C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J R Soc Interface. 2022 Aug;19(193):20220325. doi: 10.1098/rsif.2022.0325. Epub 2022 Aug 3. PMID: 35919980; PMCID: PMC9346374.
- Minaev BF, Panchenko AA. Spin–catalysis of Unsaturated Substrates Oxidation by Cofactor–free Mono– and Di–oxygenases. How Triplet Oxygen Can Overcome Spin Prohibition. Ukr J Medicine Biology and Sport. 2019 Jul; 4(6): 329-343. DOI: 10.26693/jmbs04.06.329.
- Pourshirband N, Nezamzadeh-Ejhieh A. A Z-scheme AgI/BiOI binary nanophotocatalyst for the Eriochrome Black T pho-todegradation: A scavenging agents study. Mater Res Bull. 2022; 148: 111689.
- Land JE, Raith W. Fine Structure of O2− Measured by Electron Time-of-Flight Spectroscopy. Phys Rev Lett. 1973; 30: 349-352.
- Lee J, von Gunten U, Kim JH. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ Sci Technol. 2020 Mar 17;54(6):3064-3081. doi: 10.1021/acs.est.9b07082. Epub 2020 Feb 27. PMID: 32062964.
- Derikvandi H, Nezamzadeh-Ejhieh A. Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: Effect of coupling, supporting, particles size and calcination temperature. J Hazard Mater. 2017 Jan 5;321:629-638. doi: 10.1016/j.jhazmat.2016.09.056. Epub 2016 Sep 23. PMID: 27694027.
- Pourtaheri A, Nezamzadeh-Ejhieh, A. Photocatalytic properties of incorporated NiO onto clinoptilolite nano-particles in the photodegradation process of aqueous solution of cefixime pharmaceutical capsule. Chem Eng Res Design. 2015 Dec; 104: 835–843.
- Minaev BF. Spin-orbit coupling of charge-transfer states and the mechanism for quenching singlet oxygen by amines. Theoretical and Experimental Chemistry. 1984 Jul; 20(2): 209–212.
- Lagrone R. The Great Oxygenation Event, Springer International Publishing. Cham. 2019; 129–154.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
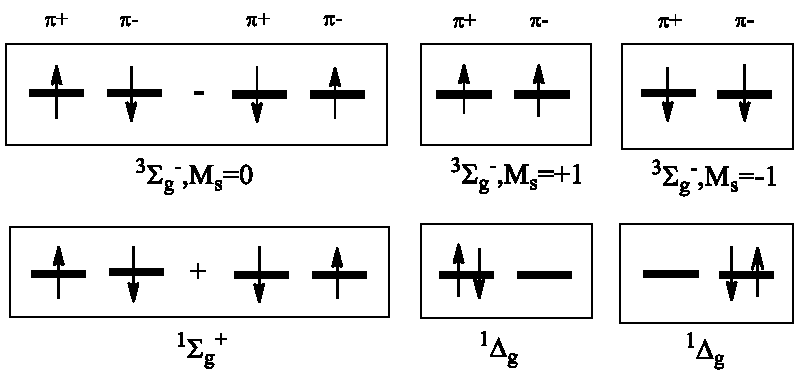

 Save to Mendeley
Save to Mendeley
