Open Journal of Plant Science
Somatic embryogenesis induction of Syzygium cumini
Mahrous H Mahrous1*, Amr El-Hawiet2, Amany E Ragab3, Hala M Hammoda2 and Fathy K EL-Fiky1
2Department of Pharmacognosy, Faculty of Pharmacy, Alexandria University, Alexandria 21521, Egypt
3Department of Pharmacognosy, Faculty of Pharmacy, Tanta University, Egypt
Cite this as
Mahrous MH, El-Hawiet A, Ragab AE, Hammoda HM, EL-Fiky FK (2023) Somatic embryogenesis induction of Syzygium cumini. Open J Plant Sci 8(1): 005-009. DOI: 10.17352/ojps.000051Copyright
© 2023 Mahrous MH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Somatic embryogenesis serves as an effective alternative system for in vitro cultivation of endangered plants (Syzygium cumini), as it allows for the propagation of plants under a controlled environment. So produce hundreds of embryos that can be used as artificial seeds. Somatic embryos of Syzygium cumini, family Myrtaceae, were induced from the calli of a sterile leaf explant in Murashige and Skoog's medium with 6 ppm 2,4-D. After the transfer of the developed calli into liquid media supplemented with the same concentration of growth regulator, all three embryonic stages (globular, heart and torpedo) were observed after 6 weeks. Liquid media with growth regulators appeared to enhance the development to torpedo-stage embryos, especially at six weeks of age cultures. 12 weeks after the transfer of the callus into liquid media, flasks containing predominantly one microscopic stage were pooled and plated on fresh solid media lacking a growth regulator, where the embryogenic calli germinated showing shoots and aerial parts. Embryogenesis production protocol is considered a good tool to save plants from extinction, especially in Syzygium cumini which is a very important anti-diabetic drug.
Introduction
The genus Syzygium, which contains around 1200 different species, is the biggest in the family Myrtaceae. In addition, Syzygium is the sixteenth-largest flowering plant genus in the world [1]. Syzygium cumini is indigenous to the Indian subcontinent [2]. According to Gajera [3], Vijay Kothari [4] and Arunpandiyan [5]; Syzygium cumini is also known as “Jambolan”, Eugenia cumini, “Jambul”, and “Kala Jamun” in India. Syzygium cumini is an endangered plant in Egypt, present only in the Antoniadis Garden in Alexandria, Egypt.
Syzygium cumini is a traditional medicinal plant. Its fruit pulp, seed, bark, and leaves showed the medicinal value and have been used in various pharmaceutical formulations for the treatment of various kinds of diseases due to therapeutic prospects. It reduces metabolic abnormalities [6] and reduces blood sugar levels significantly. An ethanol extract from a plant was found to have a dose-dependent impact on fasting blood glucose [7]. It also has analgesic [8], anti-microbial [9-11], anti-inflammatory [12], anti-oxidant activity [13], hypolipidemic [14], cardioprotective [15], antidiarrheal, antiallergic, antifertility [16], anti-clastogenic, gastroprotective, antidermatophytic, antiviral [17], antianemic [18], carminative, anti-neoplastic, radioprotective, anti-HIV [19], diuretic, antipyretic (reduce fever), anticancer, anorexigenic, antiarthritic, aphrodisiac, antiscorbutic and cytotoxic activities [20-22]. It has protective and disease-preventive health properties and also contributes to the flavor, color, texture, and aroma of the plant [23]. Carotenoids, flavonoids, sterols, phenolics, anthocyanins, and terpenes are the major phytochemicals present in the stem, fruits, bark, seed, and leaves. About 30 different phytochemical compounds have been reported in the pulp. S. cumini fruit is rich in anthocyanins, gallic acid, ellagic acid, glucoside, caffeic acid, ascorbic acid, coumaric acid, isoquercetin Maslinic acid, Corosolic acid, Betulinic acid, myricetin and kaempferol [24-26]. S. cumini fruit and leaves contain various essential oils; 82% of the total essential oils are found in the leaves. It was found that oils induce apoptosis in the HT116 human colon cancer cell by stimulating the Bax protein and the activation of caspases as well as inducing apoptosis by activating the ERK pathway [27].
Somatic embryogenesis is an in vitro biological process in which bipolar structures (somatic embryos) can be induced to form from somatic cells and regenerate into whole plants [28]. Plant regeneration through the somatic embryogenesis pathway offers an advantage over the organogenesis approach, avoiding the risk of developing chimeras [29].
Somatic embryogenesis is a technique of in vitro plant propagation where somatic cells gain the embryogenic ability to form an entire plant. Typically, somatic embryogenesis consists of two stages: first, somatic cells start dedifferentiation and then redifferentiation to gain the capacity for embryogenesis, and then embryogenic cells form somatic embryos and develop into plants. Plant regeneration by somatic embryogenesis has numerous advantages, including the potential to develop a complete plant from a single cell and the ability to produce embryos automatically and in large quantities in a bioreactor, as well as synthetic seeds that can be planted in the field. The polarity of embryos permits direct growth into plantlets without any need for the rooting stage required in organogenesis for plant regeneration. In addition, a unicellular origin can prevent chimerism, thereby reducing the burdensome screening work required during the later stages of transgenic plant development. Somatic embryogenesis resembles zygotic embryogenesis in many ways. SE is an outstanding experimental model for examining the molecular mechanisms underlying early embryogenesis.
Somatic embryogenesis has a number of benefits, including reduced material requirements, high genetic stability, reduced mutation rates, and greater regeneration rates. It is frequently used for mass propagation in vitro, cell mutation induction, cell fusion, hybrid zygotic embryo rescue, germplasm resource conservation, synthetic seed preparation, and molecularly-assisted breeding. In recent years, other characteristics of this strategy have also become popular research topics [30].
This study describes the conditions required to induce somatic embryogenesis in Syzygium cumini from a callus initiated from one piece of leaf, which produces hundreds of embryos, which are considered "artificial seeds."
Materials and methods
Aseptic plant
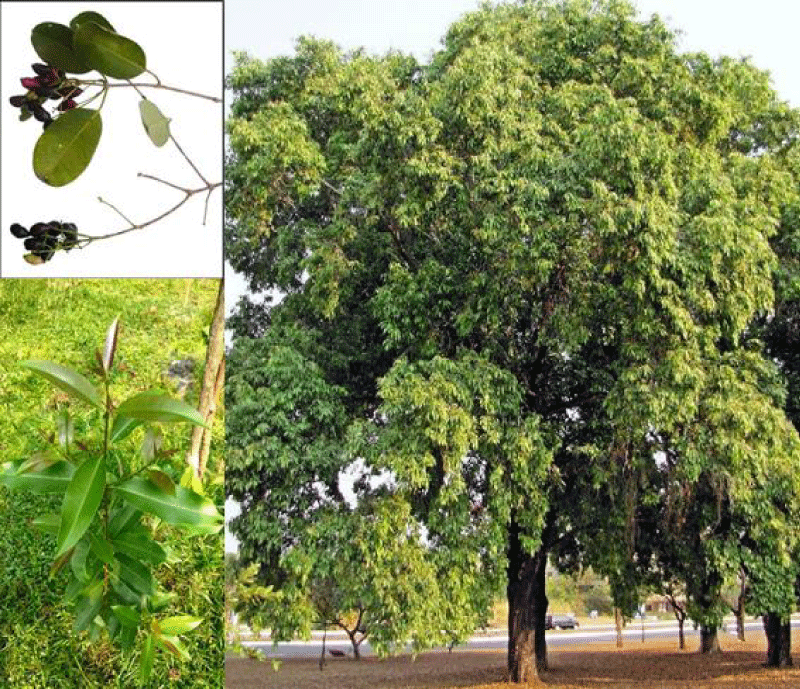
Syzygium cumini leaves (Figure 1) (obtained from Antoniadis Garden, Alexandria, Egypt) were placed in approximately 10 ml of tap water with 0.3 ml of 95% ethanol for 30 minutes to ensure wetting. The leaf is dissected into many parts. The leaf parts were then removed and sterilized in a 2.5% sodium hypochlorite solution as an effective bactericide and fungicide for 5 minutes before being rinsed with sterile distillate water [31].
Culture media
The culture medium containing Murashige & Skoog's (MS) medium (1962) was augmented with 3% (w/v) sucrose, 1% (w/v) agar, and standardized pH (6) was maintained before autoclaving at 121 °C for 18 min. Media (static culture) is dispensed in Petri dishes. The basal medium was supplied with various (1, 2, 3, 4, 5, or 6 ppm) concentrations of 2, 4-Dichlorophenoxyacetic acid (2, 4-D); or was used without growth regulators (MS). Incubation of explants was done in Petri dishes containing 10 ml of the medium under aseptic conditions and inoculated cultures were maintained for 16 hours light (33000 Lux) and 8 hours dark at 28 °C and 22 °C, respectively. The liquid culture was prepared with the same content without agar. Liquid cultures were grown in 50 ml of medium in 250 ml Erlenmeyer flasks under continuous light (8000 Lox) at 25 °C on a shaker incubator set at 78 - 80 rpm.
Callus initiation
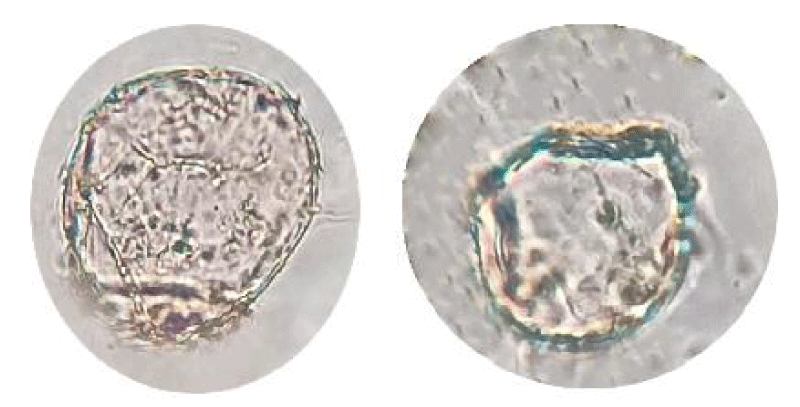
The aseptic explant (part of the leaf) was transferred to a 2,4-D static medium. After 6 weeks of incubation, only well-developed yellow-white friable (YWF) and green friable (GF) regenerating calli (3 mm pieces) (Figure 2) from the optimal concentration of 2,4-D (6 ppm) were sub-cultured to MS basal media supplied with 2,4-D (6 ppm) to increase biomass.
Embryo development
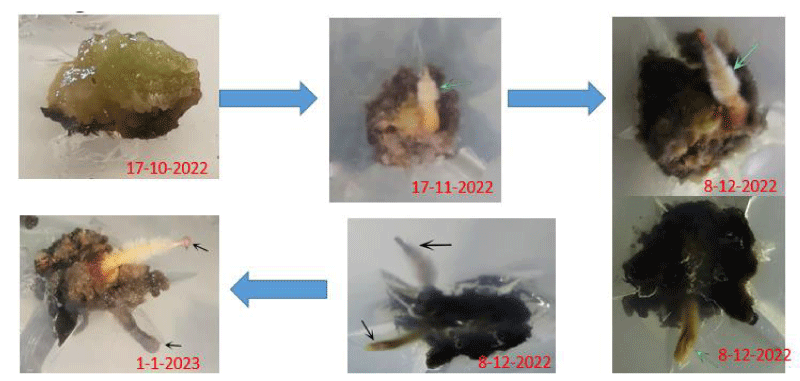
The tissues were then subcultured into liquid media of the same composition for 6 weeks and then transferred for 6 more weeks into the liquid media to promote embryo growth and development. Culture flasks containing embryos were examined microscopically after 12 weeks of growth in liquid media and were categorized into flasks containing principally globular stage embryos, heart stage embryos, or torpedo stage embryos. Each embryo category was pooled, the excess medium was decanted, and the embryos were divided into three 250 ml Erlenmeyer flasks. The tissues were then subcultured onto solid media containing no hormones (hormone free) for 4 weeks to promote embryo growth and development (Figure 3). Calli germinated in approximately 30 days to a plantlet with a shoot and root system when grown in 16 hours light (33 000 Lux) and 8 hours dark at 28 °C and 22 °C, respectively.
Results and discussion
Callus induction
In the callus induction step the visual appearance of calluses formed on 3% sucrose and 2,4-D is the most important step. Plant cell, tissue, or organ culture normally requires sucrose as a carbon source for cell proliferation and development [32,33]. The availability of sucrose in the culture medium has been found to affect somatic embryogenesis in many woody plant species [34,35]. The total number of somatic embryos was highest at 3% sucrose in media. Media with 2,4-D concentrations 1,2,3,4 and 5 ppm could not induce calli; meanwhile, media with a concentration of 6 ppm was able to induce them as it was reported that at high concentrations of synthetic auxins such as 2, 4-dicholorophenoxyacetic acid (2,4-D) induce callus [36], yet it needed much longer time to produce calluses from explants, so the optimum concentration for calli induction in each plant varies with the species of plant and type of culture [37]. In addition, it is also related to the age and size of the explant's needs [38].
Induction of somatic embryos
The calli examined microscopically after 6 weeks found globular masses of compact cells at the periphery that were approximately 0.23 mm in diameter. These peripheral cells were smaller, contained a dense cytoplasm, and were more polygonal in shape than the unorganized, highly vacuolated cells within the calli.
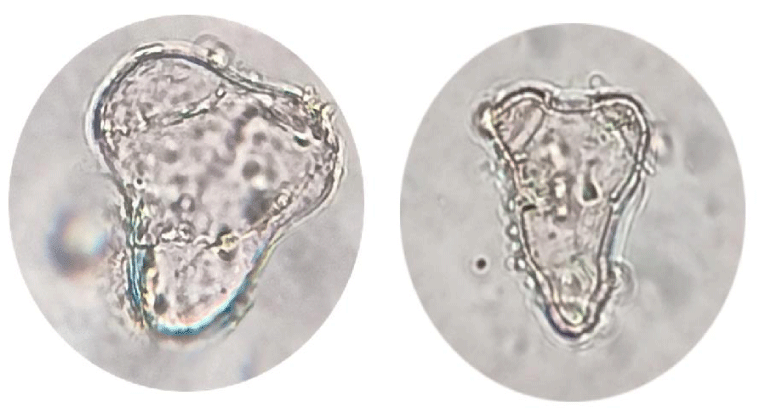
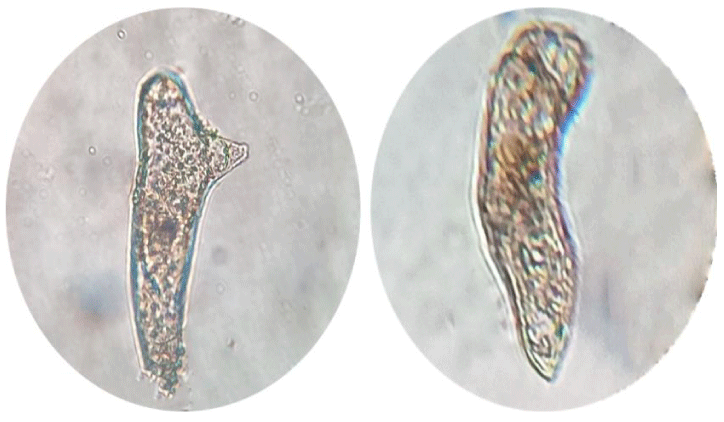
Calli subcultured into liquid media [39] rapidly developed into globular, heart, and torpedo embryos, particularly in a medium supplemented with 6 ppm 2,4-D. Globular embryos were approximately 0.4 mm in diameter and contained a multicellular suspensor at the point of attachment to the callus (Figure 4), heart-stage embryos were approximately 0.4 mm in length and 0.3mm in width (Figure 5) and torpedo-stage embryos were approximately 1.8mm length and 0.6 mm in width (Figure 6). Callus transferred from liquid media to solid plain media as it is necessary to remove plant growth regulators at the somatic embryo development and maturation steps. 2,4-D often hampers embryo development and their subsequent conversion into plants at these steps [40] after 30 days developed a shoot and root system (Figure 7).
Conclusion
Calli initiated with MS media containing 6 ppm 2,4-D and then propagated by subculture on solid MS media to increase biomass. Callus is transferred to liquid media forming a suspension culture to accelerate growth to get an embryogenic callus. Microscopical examination showed 3 embryogenic stages (globular, heart, and torpedo). Embryogenic callus was then plated on solid plain MS media that displayed germination with a germination percentage reaching nearly 25%. Since Syzygium cumini is considered an endangered plant, then embryogenesis production protocol could be adopted or mass clonal plant propagation under controlled environmental conditions.
Accordingly, it is highly recommended to try this protocol together with optimizing environmental conditions (temperature, pH, photoperiod, etc…) as well as culture conditions (explant selection, age of the explant, type of the explant, source of the explant, etc…) to get a higher rate and percentage of embryo germination.
- Madani B, Mirshekari A, Yahia EM, Golding JB, Hajivand S, Dastjerdy AM. Jamun (Syzygium cumini L. Skeels): A promising fruit for the future. Horticultural Reviews. 2021; 48: 275-306.
- Agarwala P, Gaurb PK, Tyagia N, Purib D, Kumar N, Kumard SS. An overview of phytochemical, therapeutic, pharmacological and traditional importance of Syzygium cumini. Asian J Pharmacogn. 2019; 3(1): 5-17.
- Gajera H, Gevariya SN, Hirpara DG, Patel S, Golakiya B. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black jamun (Syzygium cumini L.) landraces. Journal of Food Science and Technology. 2017; 54: 3180-3191.
- Kothari V, Seshadri S, Mehta P. Fractionation of antibacterial extracts of Syzygium cumini (Myrtaceae) seeds. Research in Biotechnology. 2011; 2(6).
- Dagadkhair AC, Pakhare KN, Todmal AD, Andhale RR. Jamun (Syzygium cumini) Skeels: a traditional therapeutic tree and its processed food products. International Journal of Pure & Applied Bioscience. 2017; 5(5): 1202-1209.
- Chhikara N, Kaur R, Jaglan S, Sharma P, Gat Y, Panghal A. Bioactive compounds and pharmacological and food applications of Syzygium cumini–a review. Food & Function. 2018; 9(12): 6096-6115.
- Mulkalwar S, Kulkarni V, Deshpande T, Bhide H, Patel A, Tilak A. Antihyperglycemic activity of Syzygium cumini (jamun) in diabetic rats. J Pharma Res Int. 2021; 33: 12-19.
- Rauf A, Al-Awthan YS, Khan IA, Muhammad N, Ali Shah SU, Bahattab O, Al-Duais MA, Sharma R, Rahman M. In Vivo Anti-Inflammatory, Analgesic, Muscle Relaxant, and Sedative Activities of Extracts from Syzygium cumini (L.) Skeels in Mice. Evidence-Based Complementary and Alternative Medicine. 2022.
- Remashree A, Thomas V, Nabeesa E, Neelakandan N, Nandakumar S. Plant regeneration through kalus culture of Syzyngium cumini. L Plant Cell Biotechnol Mol Biol. 2003; 4: 197-200.
- Santos CA, Almeida FA, Quecán BX, Pereira PA, Gandra KM, Cunha LR, Pinto UM. Bioactive properties of Syzygium cumini (L.) skeels pulp and seed phenolic extracts. Frontiers in Microbiology. 2020; 11: 990.
- Sharma R, Oberoi K, Sharma K, Nagraik R, Kumar D, Sharma A, Sharma S. Bioactive compounds and ethnomedicinal uses of Syzygium cumini (L.) Skeels-A comprehensive review. The Annals of the University Dunarea de Jos of Galati. Fascicle VI-Food Technology. 2020; 44(1): 230-254.
- Qamar M, Akhtar S, Ismail T, Yuan Y, Ahmad N, Tawab A, Ismail A, Barnard RT, Cooper MA, Blaskovich MA. Syzygium cumini (L.), Skeels fruit extracts: in vitro and in vivo anti-inflammatory properties. Journal of Ethnopharmacology. 2021; 271: 113805.
- Ahmed R, Tariq M, Hussain M, Andleeb A, Masoud MS, Ali I, Mraiche F, Hasan A. Phenolic contents-based assessment of therapeutic potential of Syzygium cumini leaves extract. PloS one. 2019; 14(8): e0221318.
- Ravi K, Rajasekaran S, Subramanian S. Antihyperlipidemic effect of Eugenia jambolana seed kernel on streptozotocin-induced diabetes in rats. Food and Chemical Toxicology. 2005; 43(9): 1433-1439.
- Mastan S, Chaitanya G, Latha TB, Srikanth A, Sumalatha G, Kumar KE. Cardioprotective effect of methanolic extract of Syzygium cumini seeds on isoproterenol-induced myocardial infarction in rats. Der Pharmacia Lettre. 2009; 1(1): 143-149.
- Srivastava S, Chandra D. Pharmacological potentials of Syzygium cumini: a review. J Sci Food Agric. 2013 Jul;93(9):2084-93. doi: 10.1002/jsfa.6111. Epub 2013 May 15. PMID: 23460190.
- Katiyar D, Singh V, Ali M. Recent advances in pharmacological potential of Syzygium cumini: A review. Advances in Applied Science Research. 2016; 7(3): 1-12.
- Khodavirdipour A, Zarean R, Safaralizadeh R. Evaluation of the anti-cancer effect of Syzygium cumini ethanolic extract on HT-29 colorectal cell line. Journal of Gastrointestinal Cancer. 2021; 52: 575-581.
- Khan AS, Khan AS. Woody plants with possible anti-HIV activity. Medicinally Important Trees. 2017; 109-131.
- Kumar A, Ilavarasan R, Jayachandran T, Decaraman M, Aravindhan P, Padmanabhan N, Krishnan M. Phytochemicals investigation on a tropical plant, Syzygium cumini from Kattuppalayam, Erode district, Tamil Nadu, South India. Pakistan Journal of Nutrition. 2009; 8(1): 83-85.
- Peixoto MPG, Freitas LA. Spray-dried extracts from Syzygium cumini seeds: physicochemical and biological evaluation. Revista Brasileira de Farmacognosia. 2013; 23(1): 145-152.
- Aqil F, Munagala R, Jeyabalan J, Joshi T, Gupta RC, Singh IP. The Indian blackberry (Jamun), antioxidant capacity, and cancer protection. Cancer, Elsevier. 2014; 101-113.
- Panghal A, Patidar R, Jaglan S, Chhikara N, Khatkar SK, Gat Y, Sindhu N. Whey valorization: current options and future scenario–a critical review. Nutrition & Food Science. 2018; 48(3): 520-535.
- Sowjanya K, Swathi J, Narendra K, Satya AK. A review on phytochemical constituents and bioassay of Syzygium cumini." International Journal of Natural Product Science. 2013; 3(2): 1-11.
- Duyen Vu TP, Quan Khong T, Nguyet Nguyen TM, Kim YH, Kang JS. Phytochemical profile of Syzygium formosum (Wall.) Masam leaves using HPLC-PDA-MS/MS and a simple HPLC-ELSD method for quality control. J Pharm Biomed Anal. 2019 May 10;168:1-12. doi: 10.1016/j.jpba.2019.02.014. Epub 2019 Feb 11. PMID: 30776566.
- Borba LA, Wiltenburg VD, Negri G, Ibe MB, dos Santos L, Mendes FR. In vitro inhibition of acetylcholinesterase and monoamine oxidase by Syzygium cumini leaves extract and preliminary assessment in animal models. South African Journal of Botany. 2022; 146: 553-563.
- Choi YH, Kong KR, Kim YA, Jung KO, Kil JH, Rhee SH, Park KY. Induction of Bax and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int J Oncol. 2003 Dec;23(6):1657-62. PMID: 14612938.
- Elhiti M, Stasolla C. Transduction of Signals during Somatic Embryogenesis. Plants (Basel). 2022 Jan 11;11(2):178. doi: 10.3390/plants11020178. PMID: 35050066; PMCID: PMC8779037.
- Raza G, Singh MB, Bhalla PL. Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars. Plants (Basel). 2019 Dec 25;9(1):38. doi: 10.3390/plants9010038. PMID: 31881730; PMCID: PMC7020241.
- Yan R, Sun Y, Sun H. Current status and future perspectives of somatic embryogenesis in Lilium. Plant Cell, Tissue and Organ Culture (PCTOC). 2020; 143: 229-240.
- Omidi M, Yadollahi A. Factors affecting in vitro propagation efficiency of three genotype of Rosa damascena. Journal of Biology and Nature. 2016; 78-87.
- Karhu ST. Sugar use in relation to shoot induction by sorbitol and cytokinin in apple. Journal of the American Society for Horticultural Science. 1997; 122(4): 476-480.
- Fuentes SR, Calheiros MB, Manetti-Filho J, Vieira LG. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue and Organ Culture. 2000; 60: 5-13.
- Canhoto JM, Lopes ML, Cruz GS. Somatic embryogenesis and plant regeneration in myrtle (Myrtaceae). Plant Cell, Tissue and Organ Culture. 1999; 57: 13-21.
- Al-Shara B, Taha RM, Mohamad J, Elias H, Khan A. Somatic Embryogenesis and Plantlet Regeneration in the Carica papaya L. cv. Eksotika. Plants (Basel). 2020 Mar 12;9(3):360. doi: 10.3390/plants9030360. PMID: 32178429; PMCID: PMC7154855.
- Yaacob JS, Ramli MA, Abd Rahim MH, Marie A. Comparative Analysis on the Role of 2, 4-dichlorophenoxyacetic Acid in the Expression of Bioactive Compounds in Callus of Capsicum frutescens. Sains Malaysiana. 2022; 51(10): 3171-3182.
- Kok T, Daniel X, Soegiono S. Induction of Callus Cultures from the Leaves of Syzygium cumini (Linn.) Skeels in Woody Plant Medium with Variations of Growth Hormones. ICoSI 2014: Proceedings of the 2nd International Conference on Sustainable Innovation, Springer. 2017.
- Prakash M, Gurumurthi K. Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell, Tissue and Organ Culture (PCTOC). 2010; 100: 13-20.
- Woo HA, Ku SS, Jie EY, Kim H, Kim HS, Cho HS, Jeong WJ, Park SU, Min SR, Kim SW. Efficient plant regeneration from embryogenic cell suspension cultures of Euonymus alatus. Sci Rep. 2021 Jul 23;11(1):15120. doi: 10.1038/s41598-021-94597-4. PMID: 34301990; PMCID: PMC8302629.
- Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B. Induction of somatic embryogenesis as an example of stress-related plant reactions. Electronic Journal of Biotechnology. 2010; 13(1): 12-13.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
